Co-administration of a polysaccharide with a chemotherapeutic agent for the treatment of cancer
a polysaccharide and cancer technology, applied in the field of cancer treatment, can solve the problems of the efficacy of a particular dosage to target and kill tumor cells, and the toxic effect of a particular dosage, so as to improve the tolerance of the patient to the agent, and improve the effect of the therapeutic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Structure of Galactomannan
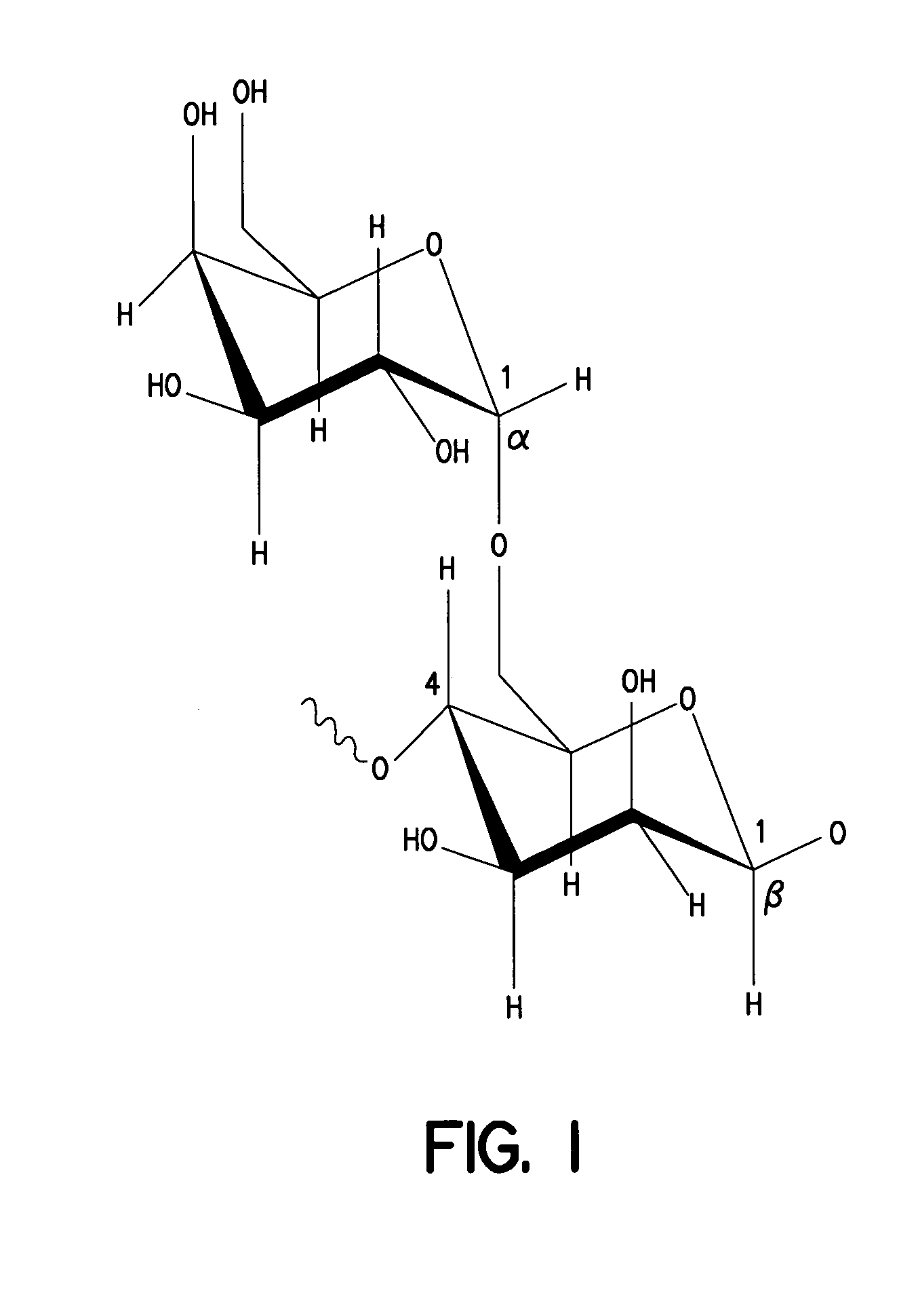
[0114] The galactomannan oligomer of the present invention is a polysaccharide. In one aspect it has an average molecular weight of about 48,000 D. Shown below is the acceptable chemical nomenclature and structural formula for the galactomann of the present invention. Also shown is the stereochemical configuration.
[0115] Full chemical name: (((1,4)-linked β-D-mannopyranose)17-((1,6)-linked-β-D-galactopyranose) 10) 12).
[0116] A backbone composed of linear (1→4)-β-D-Mannopyranosyl units, to which single β-D-Galactopyranosyl is attached by (1→6) linkage as illustrated below:
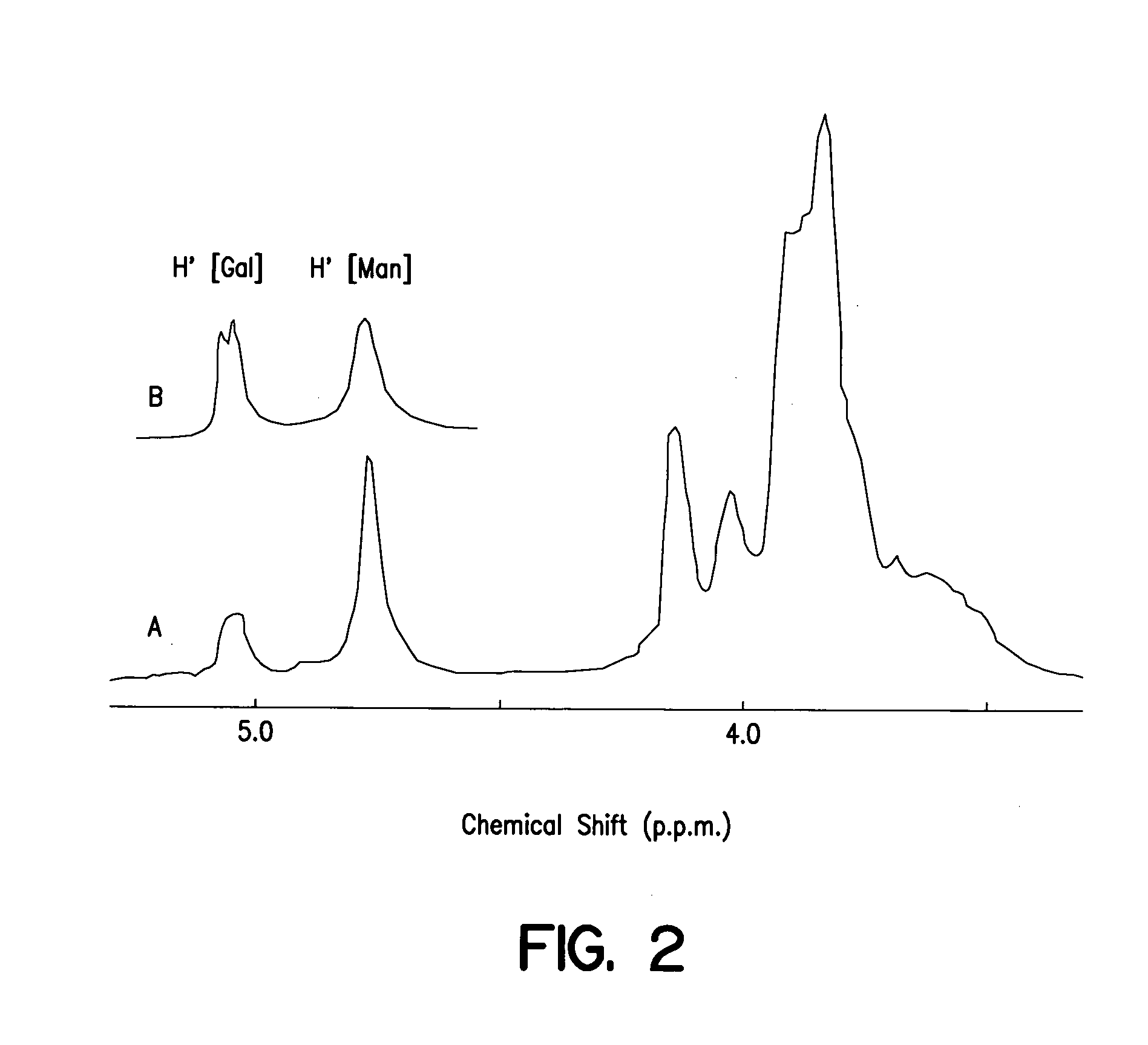
[0117]FIG. 2 shows the structure of the galactomannan polysaccharide of the present invention as determined by Nuclear Magnetic Resonance (NMR). The galactomannan of the present invention was compared to galactomannan from Carob (locust bean) gum. An easy identification of the two principal sugar residues, that is mannose (Man) and galactose (Gal), comes from two peaks, at 4.8 p.p.m. (...
example 2
[0134] Purification and Manufacturing Process
[0135] Shown in FIG. 5 is a flow chart of an example for a purification and manufacturing process for a galactomannan of the present invention. High grade Guar gum is dissolved in warm water at 1% at 45° C. for 2 hr. The pH is reduced to 2.2 with 1 M HCl and solution is heated to 95° C. for ˜2 hours. Then pH is adjust to 5.8 with 1 M NaOH. The solution is then cool to 20° C. and filter with glass filter. Next CuSO4 / Na-K tartrate is added and the precipitate is collected on 200 mesh filter, wash with water solution and than washed in 5% HCl in 96% EtOH. Than washed with 75% EtOH and twice with 96% EtOH. And finally vacuum freeze-dried as white solids. Galactomannan, from a readily available source (e.g., Guar gum), was selected for process optimization and manufacturing. The soluble galactomannan oligomer was tested in-vivo (in animals) for both efficacy and overall reduction of toxicity.
[0136] The manufacturing process described above p...
examples 3-5
[0138] Efficacy Studies
[0139] The galactomannan of the present invention is a galactomannan derivative comprising exposed galactose moieties attached to a mannose backbone. The compound is thought to interact with galactose-binding lectins or galectins that are generally located on cell surfaces. Lectins are carbohydrate-binding proteins, typically located on the cell surface, which mediate various types of cellular interactions. It is generally accepted that lectins mediate many biological recognition events in plants and in animal tissues, and in tumor cell lines. Lectins play a role in cell-cell adhesion, and in the organization of the extracellular matrix. At the cell surface, lectins can act as receptors involved in selective intercellular adhesion and cell migration, recognition of circulating glycoproteins, and modulation of cell-cell and cell-matrix interactions. Galectins are members of a family of β-galactoside-binding lectins with related amino acid sequences. Galectins ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap