Microparticle protection of therapeutic agents
a technology of therapeutic agents and microparticles, applied in the direction of bacteria material medical ingredients, catheter, biocide, etc., to achieve the effect of reducing the pharmaceutical effectiveness of the pharmaceutically active agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0073] A CMV-LacZ adenovirus (i.e., an adenoviral vector driven by a cytomegalovirus promoter and β-galactosidase reporter gene based on the LacZ enzyme) is used as a stock virus in this example.
[0074] An adenoviral solution having an AdCMV-LacZ titer of 1×109 functional units per milliliter (fu / ml) in PBS (− / −) was prepared.
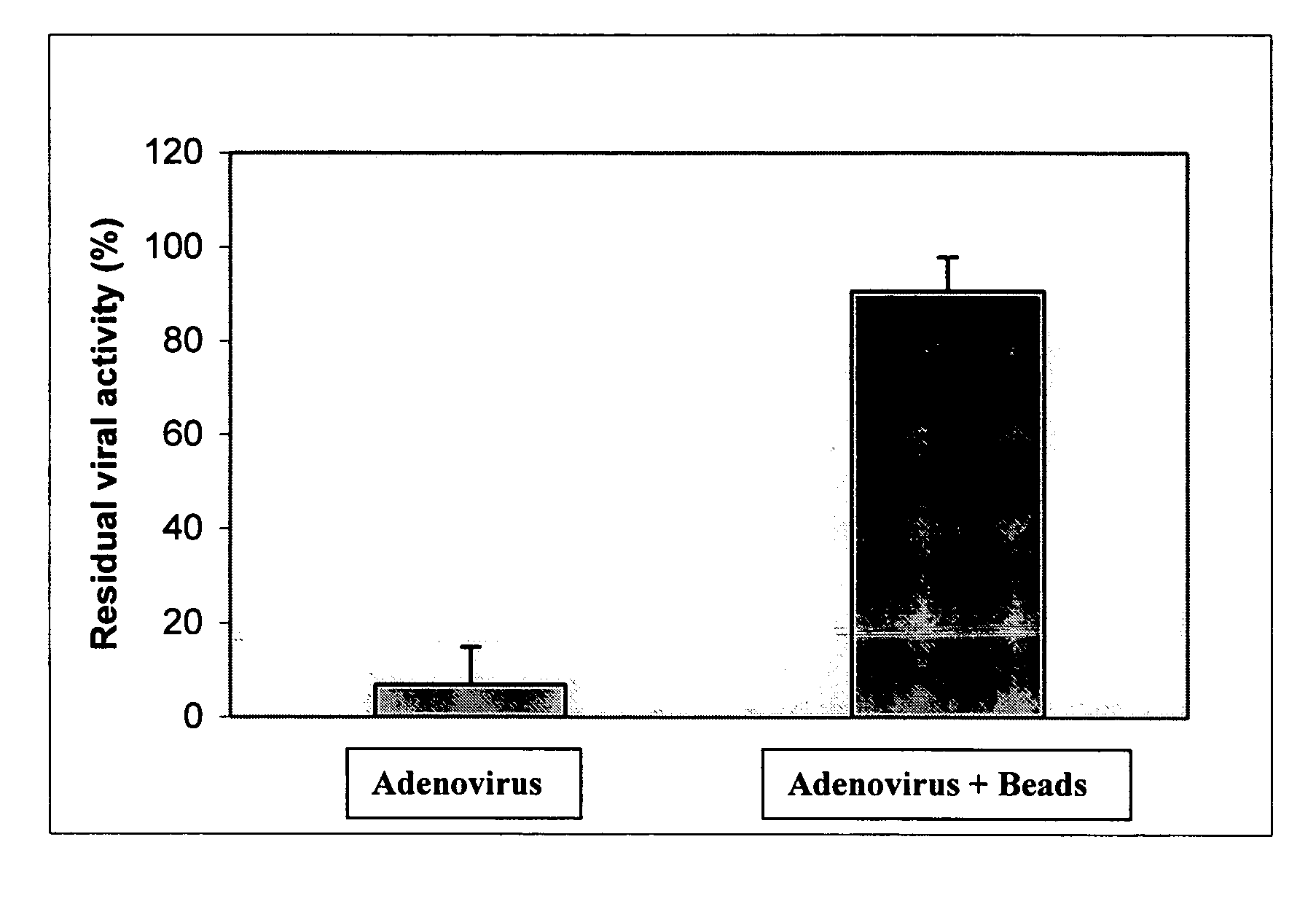
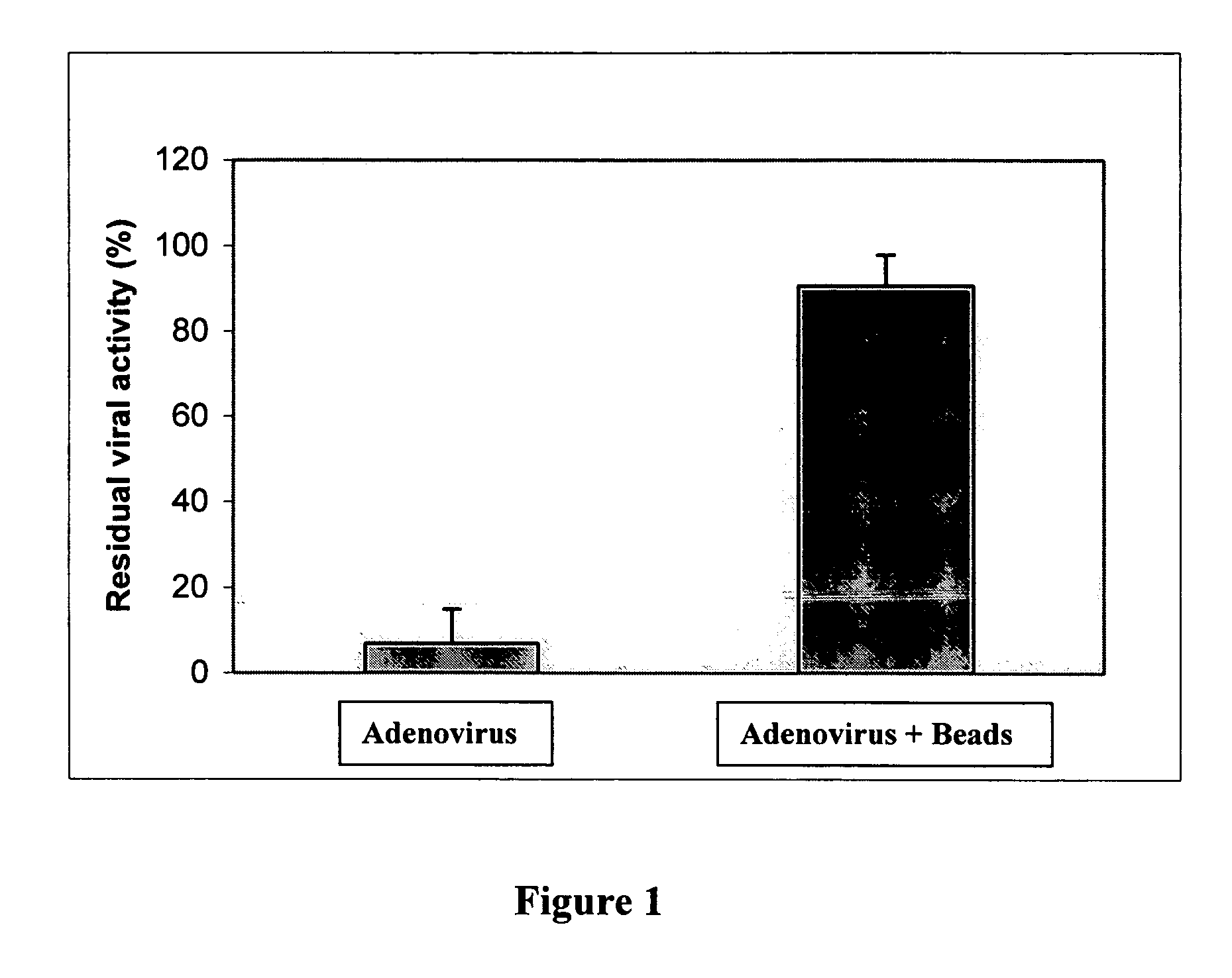
[0075] At the same time a suspension is prepared by combining the following: (a) 90 vol % adenoviral solution at a titer appropriate to give a final titer of 1×109 fu / ml and (b) 10 vol % Fluoresbrite™ YG Microspheres from Polysciences Inc., which contains 1.0-micron fluorescent polystyrene beads at a concentration of 2.5% solids in water. This combination results in a suspension that contains a final adenovirus titer of 1×10 fu / ml as well as 0.25% solids as microspheres (due to the 10:1 dilution of the bead solution).
[0076] Boston Scientific Corporation Stiletto™ direct injection catheters, which have a proximal portion formed from heat-treated stainless stee...
example 2
[0079] Efficacy of adenovirus delivery from a standard needle was performed in a mouse model. An adenoviral solution and an adenoviral suspension containing beads (10 vol % Fluoresbrite™ YG Microspheres), each having an AdCMV-LacZ titer of 109fu / 50 μl, were prepared. Mice were anesthetized and a left thoracotomy was used to expose the heart. Direct epicardial injections (1×50 μl) were made into the left ventricle. Animals were sacrificed 7 days following the procedure. Whole hearts were retrieved and assayed by spectroscopic absorption at 420 nm to quantify the total amount of beta-galactosidase expressed. Absorption data are given below on the basis of both sample size (150 μl) and mg protein (determined using a standard protein assay). These data indicate that beta-galactosidase expression is higher for the injections containing beads.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| dimension | aaaaa | aaaaa |
| superelastic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com