Vaccine immunotherapy for immune suppressed patients
a technology for immune suppressed patients and vaccines, applied in the field of vaccine immunotherapy, can solve the problems of sporadic and generally minor successful efforts, inability to immunize on a consistent basis, and inability to achieve consistent immunization, so as to promote differentiation and maturation of immature dendritic cells, and restore t-cell immunity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0094] All steps relating to cell culture are performed under sterile conditions. General methods of cellular immunology not described herein are performed as described in general references for Cellular immunology techniques such as Mishell and Shiigi (Selected Methods in Cellular Immunology, 1981) and as are known in the art.
Preparation of Natural Cytokine Mixture (NCM)
[0095] The buffy coat white cells of human blood from multiple HIV− negative hepatitis virus-negative donors is collected. In an alternative embodiment, animals could be the cell source for veterinary uses. The cells from the donors are pooled and layered on ficoll hypaque gradients (Pharmacia) to yield lymphocytes free of neutrophils and erythrocytes. Alternative methods could be used that would result in the same starting lymphocyte population as are known in the art.
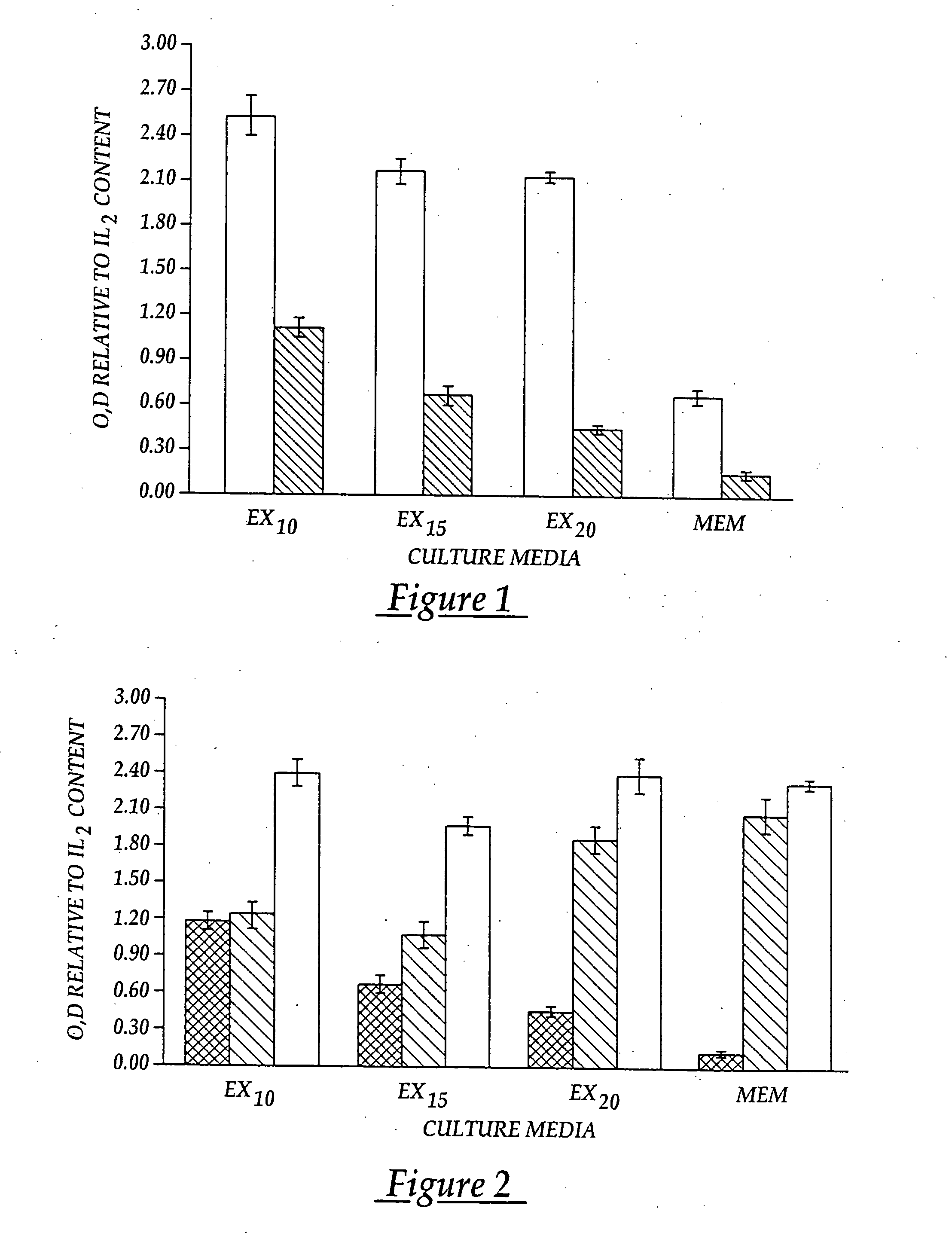
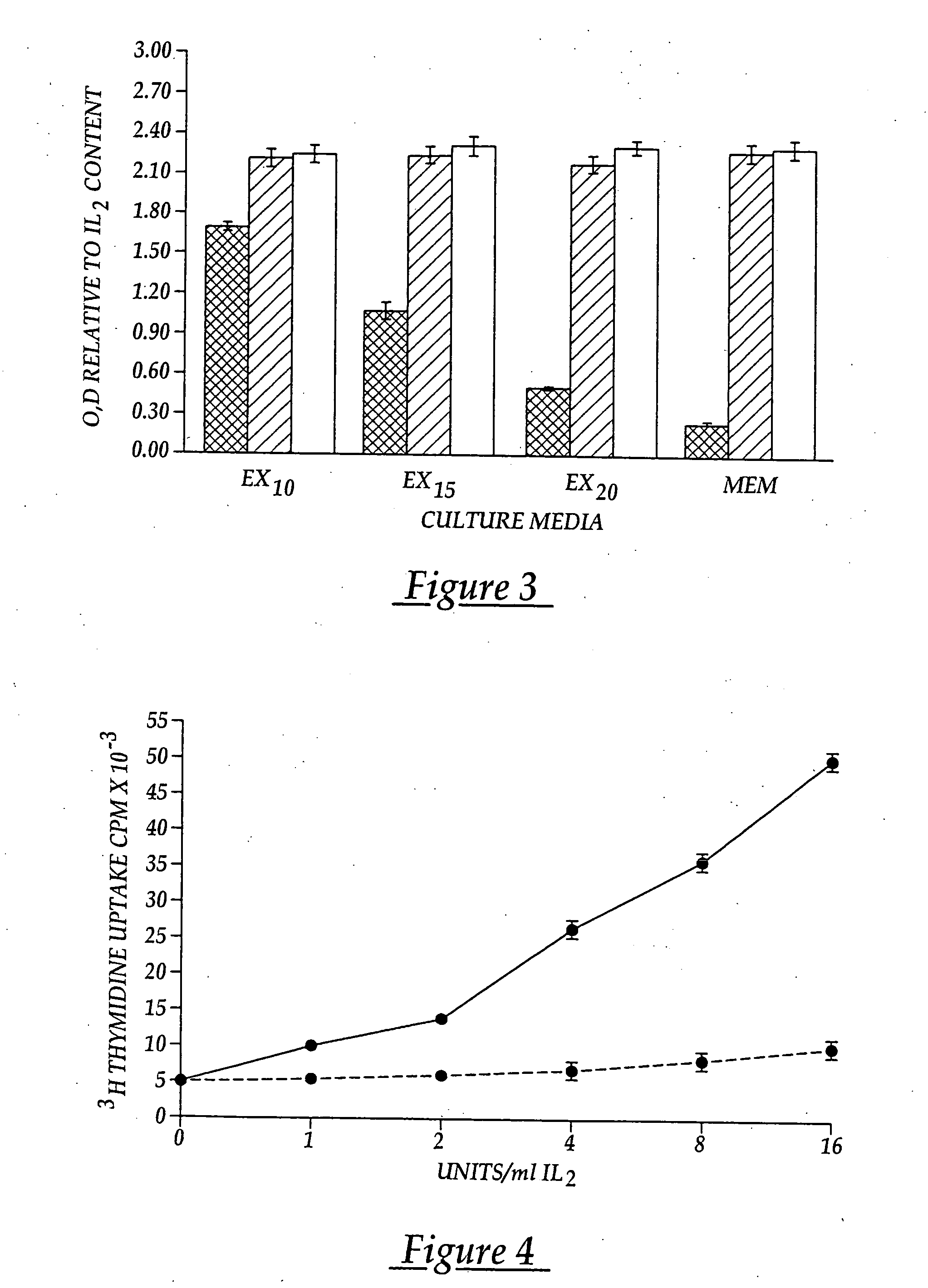
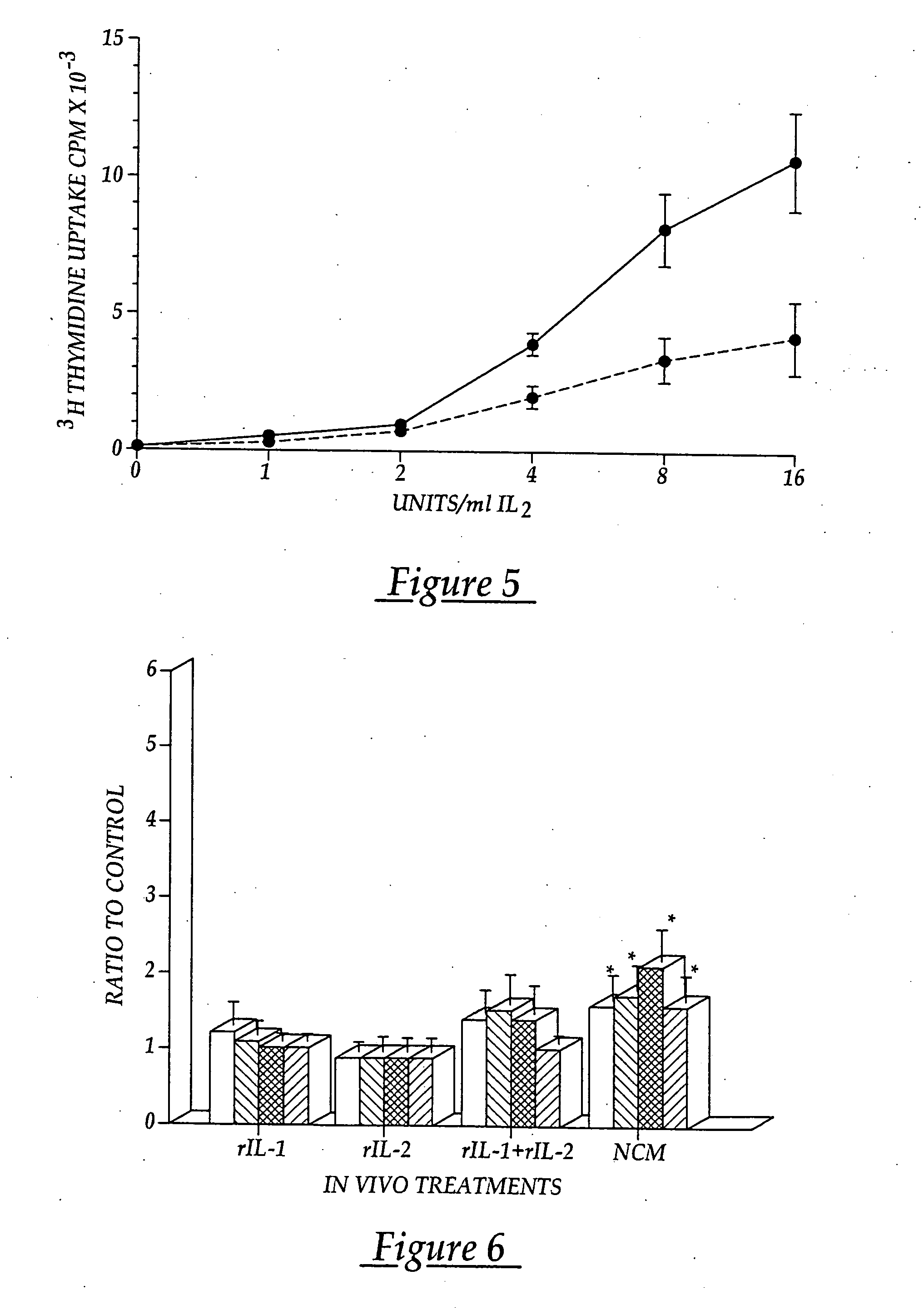
[0096] The lymphocytes are washed and distributed in X vivo-10 media (Whittaker Bioproducts) to surface activated cell culture flasks for selecti...
example2
[0135] There is shown that local perilymphatic injections in the neck having NCM plus low dose cyclophosphamide, indomethacin, and zinc and induced clinical regressions in a high percentage of patients with squamous cell head and neck cancer (H&NSCC) (Hadden J W, et al., Arch Otolaryngol Head Neck Surg. 120:395-403, 1994; Meneses A, et al., Arch Pathol Lab Med 122:447-454, 1998; Barrera J, et al., Arch Otolaryngol Head Neck Surg 126:345-351, 2000) with evidence of improved, recurrence-free survival. Overall, including minor response (25%-50%) tumor shrinkage and reduction of tumor in pathological specimens, over 90% responded and the majority had greater than 50% tumor reduction.
[0136] These responses were speculated to be mediated by immune regression since both B and T lymphocytes were observed infiltrating the tumors. The therapy was not associated with significant toxicity.
[0137] Several unpublished observations serve to document this speculation and lead to the present invent...
example 3
[0147] Two patients were treated with lymphoma of the head and neck.
[0148] The patients included were those with head and neck cancer who agreed to participate in the protocol. The following scheme was followed:
[0149] Before treatment, the patients were skin-tested with NCM 0.1 ml subcutaneously in the forearm, the region was marked, and 24 hrs. later the test was read. The test was considered positive if the induction and erythema was equal or larger than 3 mm.
[0150] Each cycle of NCM was for 21 days as follows: [0151] Day 1: Low dose cyclophosphamide (300 mg / m2i.v.) [0152] Day 1-21: Indomethacin 25 mg p.o. 3 times daily Zinc sulfate 50 mg p.o. once daily [0153] Day 3-12: NCM 200 units five as 1 ml subcutaneously perilymphatic in the neck.
Case #1
[0154] The patient was a 23-year-old male who presented on with a prior history of three months of the presence of a tumor on the left submaxillary region, with no other symptoms. In the emergency room, he was found to have lymph aden...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com