Methods for treatment of diabetes using peptide analogues of insulin
a technology of peptide analogues and insulin, which is applied in the direction of peptide/protein ingredients, extracellular fluid disorder, metabolic disorders, etc., can solve the problems of generating neutralizing antibodies, difficulty in maintaining proper dosage, and variety of hypoglycemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Peptides
[0051] This Example illustrates the synthesis of representative peptide analogues.
[0052] Peptides were synthesized by solid phase methodology on a peptide synthesizer (Beckman model 990). Peptides with an amidated carboxyl-terminus were prepared with a p-methylbenzhydrylamine resin (MBHA resin); for peptides with a free carboxyl-terminus, a Merrifield resin coupled with the appropriately protected amino acid was used. Both resins were obtained from Bachem Fine Chemicals (Torrance, Calif.). Derivatized amino acids (Bachem Fine Chemicals) used in the synthesis were of the L-configuration unless specified otherwise, and the N-alpha-amino function protected exclusively with the t-butyloxycarbonyl group. Side-chain functional groups were protected as follows: benzyl for serine and threonine; cyclohexyl for glutamic acid and aspartic acid; tosyl for histidine and arginine; 2-chlorobenzyloxycarbonyl for lysine and 2-bromobenzyloxycarbonyl for tyrosine. Coupling of ...
example 2
Long-Term T Cell Lines
[0053] This Example illustrates the preparation of long-term insulin-specific NOD T cell lines.
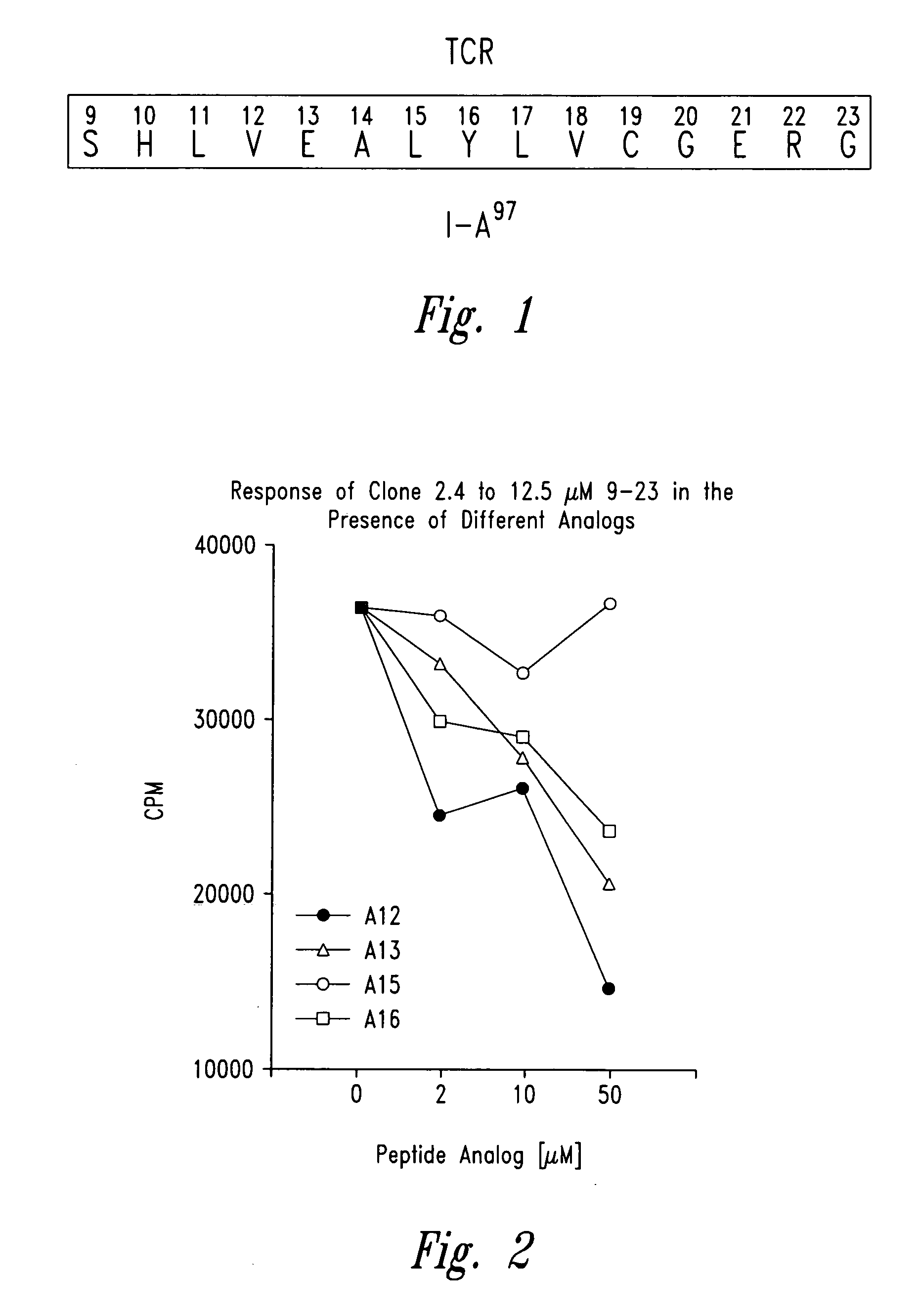
[0054] Insulin specific NOD T cell lines were established by culturing lymphocytes isolated from islet-infiltrating populations by in vitro stimulation with either porcine insulin at 25 μg / ml and irradiated NOD islet cells in the presence of irradiated NOD spleen cells as antigen presenting cells and cytokines. To obtain the infiltrating lymphocytes the following procedures were performed (see Wegmann et al., Eur. J. Immunol. 24:1853, 1994): the pancreas from the NOD mouse was digested with collagenase and individual islets were isolated manually. The infiltrating lymphocytes were then obtained by mild trypsin digestion of the islets. The insulin specific T cell lines or clones were propagated by serial stimulation in the presence of NOD spleen cells, porcine insulin and lymphokines. Clones were obtained by limiting dilution of the B chain (9-23) specific T cell lin...
example 3
Effect of Peptide Analogues on Proliferation of Insulin-Specific NOD T Cell Clones
[0055] This Example illustrates the effect of representative peptide analogues on T cell proliferation.
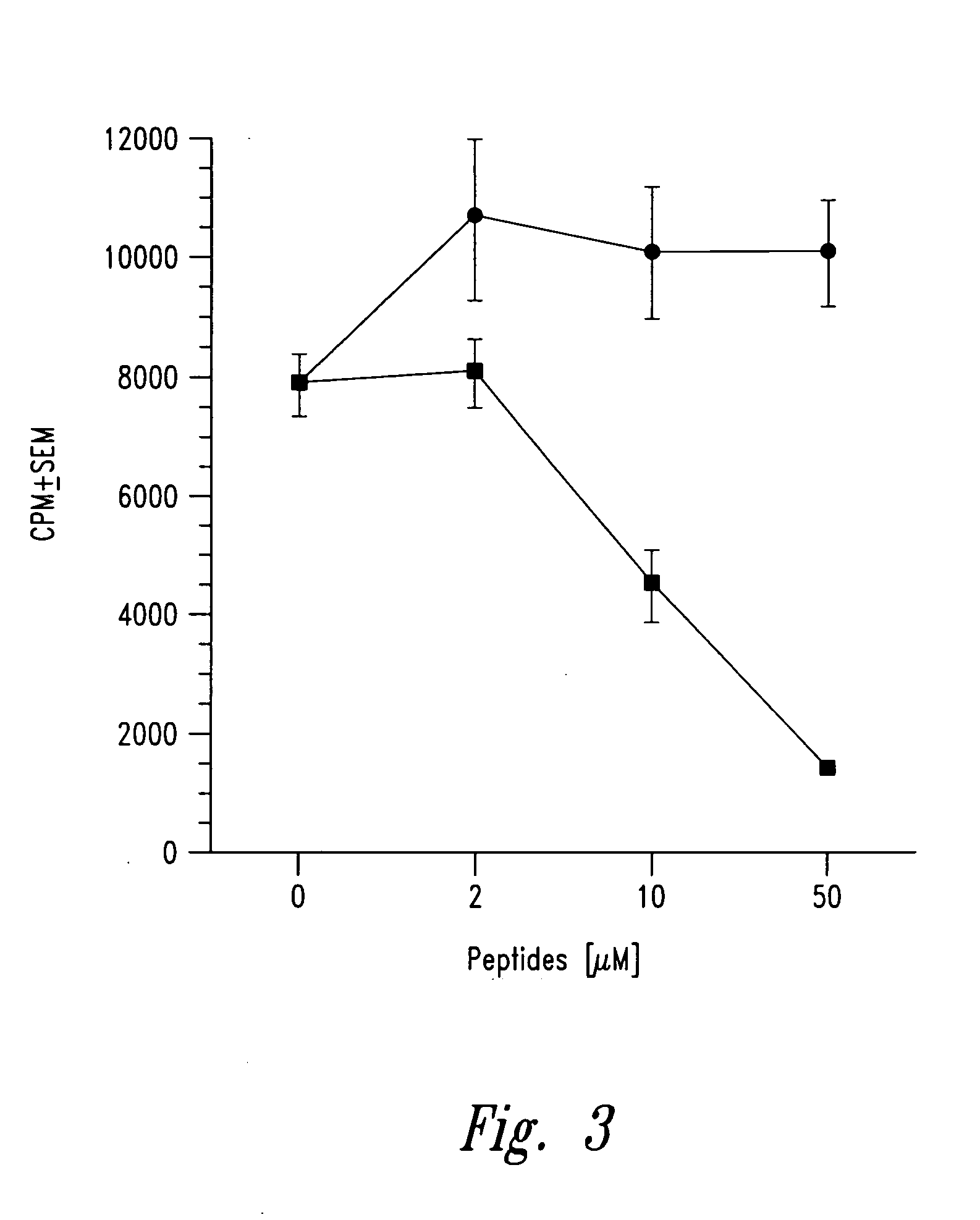
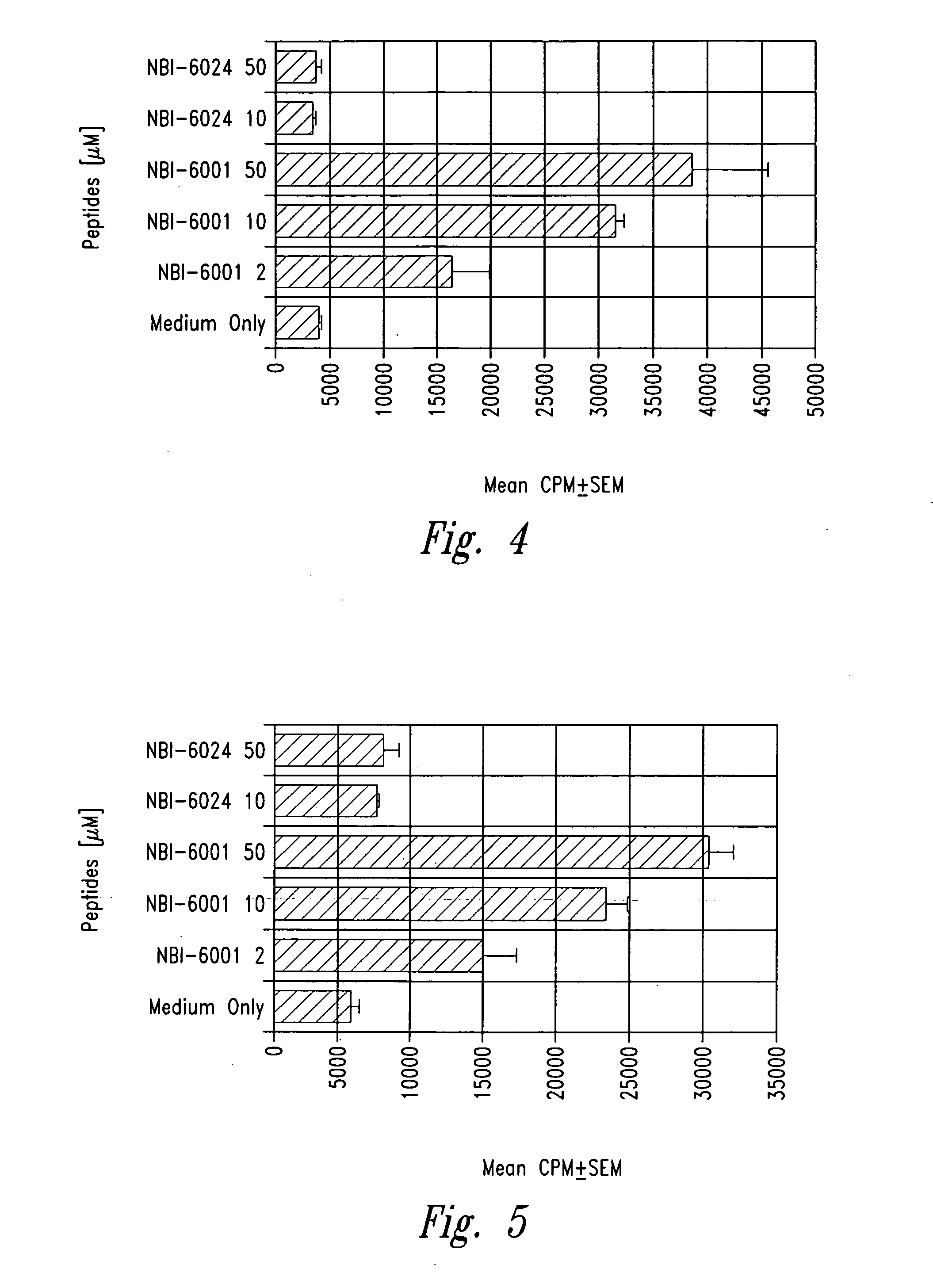
[0056] Insulin B chain (9-23) (SEQ ID NO:2) specific mouse (NOD) T cell clones were isolated from infiltrated islets as described in Example 2. Peptide analogues with single alanine substitutions were prepared as described in Example 1. The effect of each analogue on T cell proliferation was then evaluated using an assay performed in 96-well flat bottom microtiter plates (see Daniel et al., Eur. J. Immunol. 25:1056, 1995). Briefly 25,000 T cell clones along with 1 million irradiated NOD spleen cells were cultured in the presence of 50 μg / ml of insulin B chain 9-23 peptide or any of the alanine substituted peptides listed below in triplicate sets. The plates were incubated for a total of 72 hours in 7% carbon dioxide atmosphere with a pulse of 1 μCi / well of tritiated thymidine for the last 6-8 hours ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compositions | aaaaa | aaaaa |
| liquid | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com