Aneurysm embolic device with an occlusive member
an embolic device and aneurysm technology, applied in the field of catheter-based implantable medical devices, can solve the problems of affecting the healing effect of the aneurysm, and affecting the healing effect of the aneurysm, and achieve the effect of filling the aneurysm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
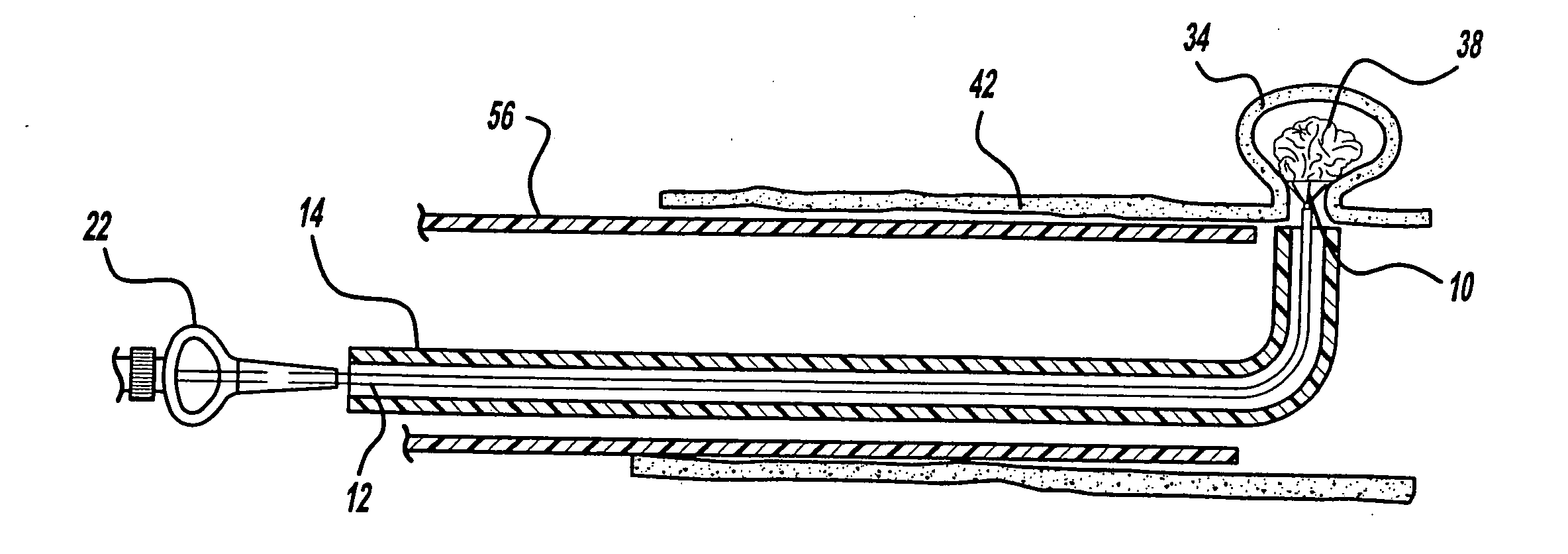
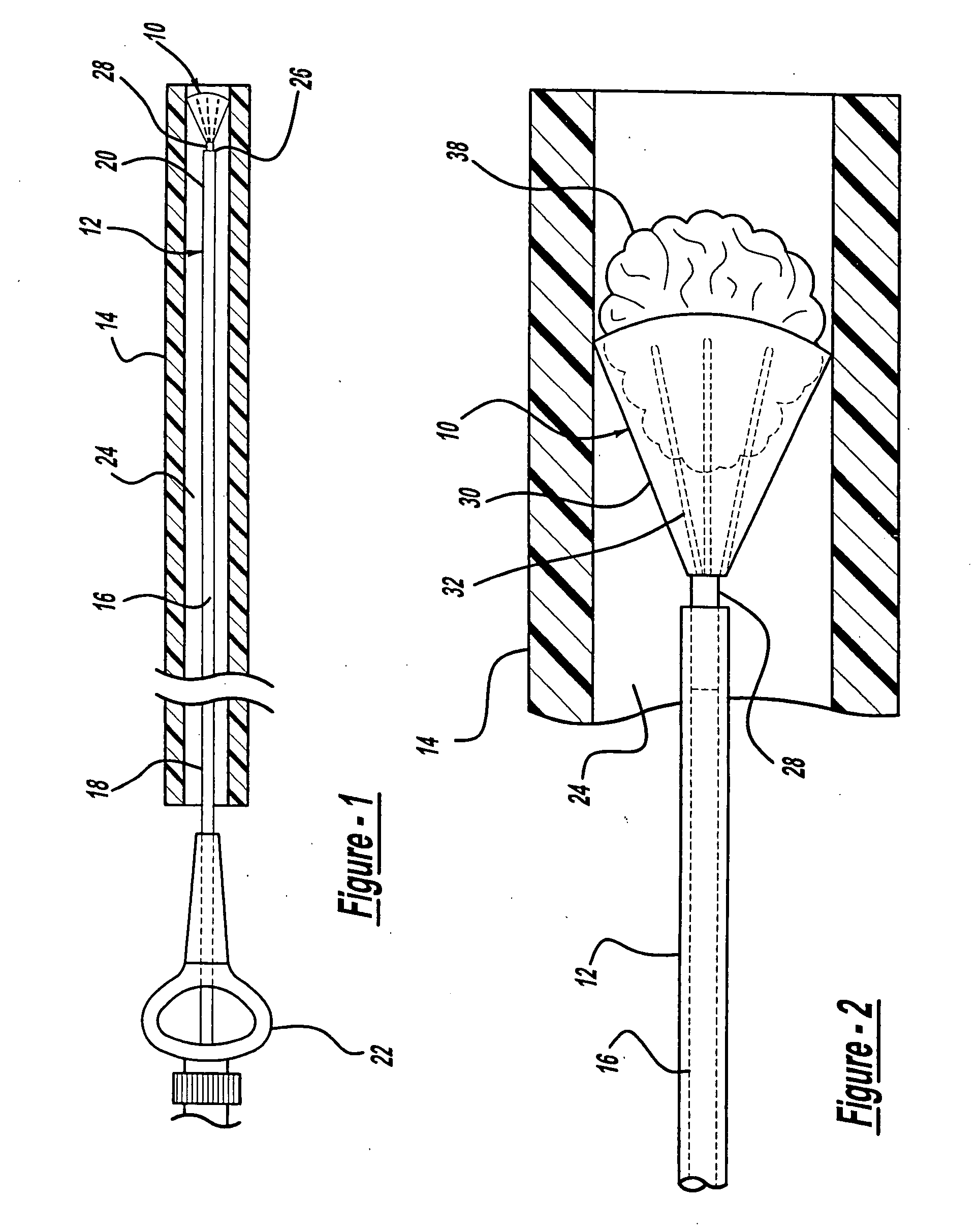
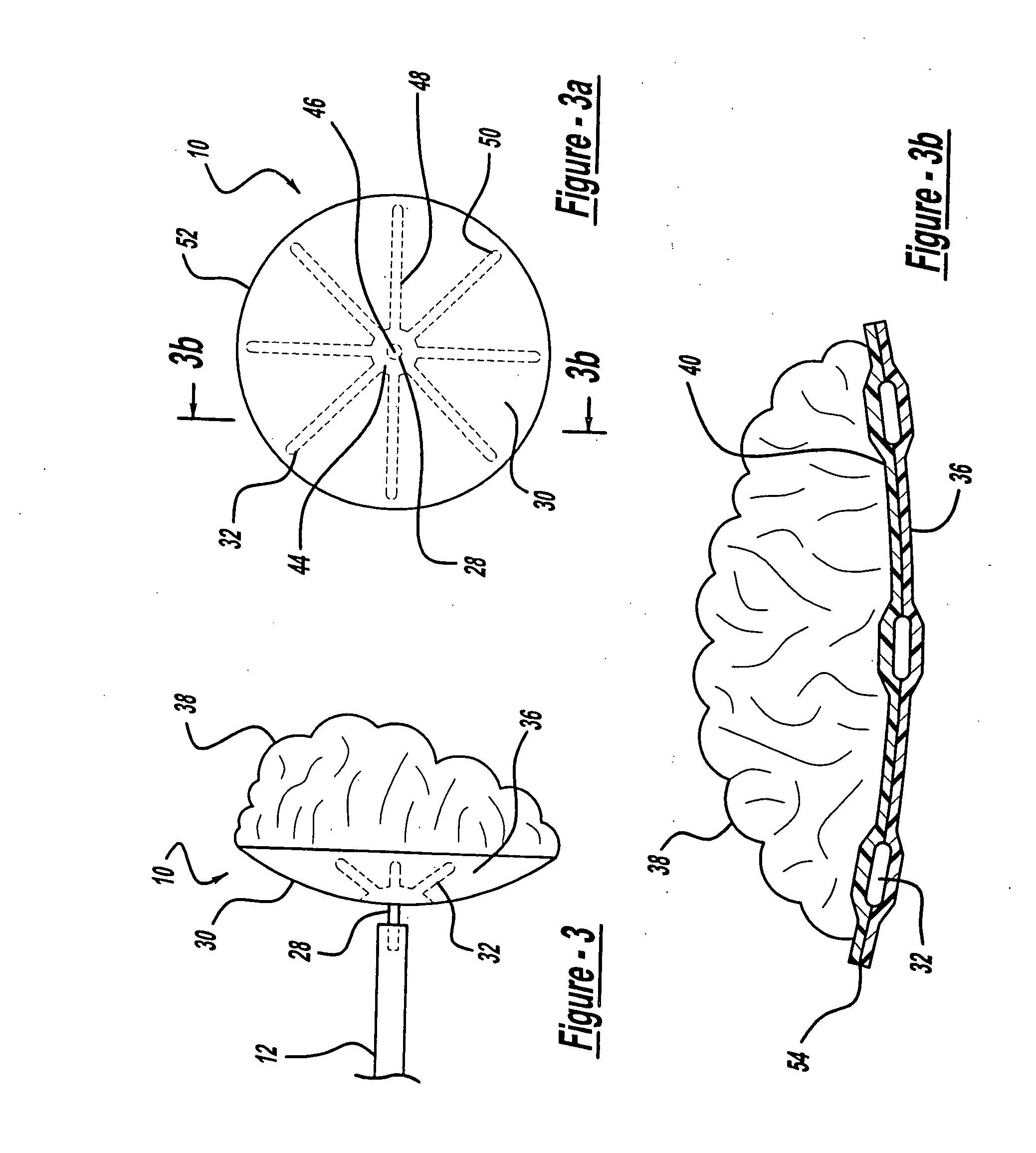
[0028]FIG. 1 illustrates an aneurysm embolic device 10, a deployment catheter 12, and a sheath 14 in accordance with the present invention. The deployment catheter 12 is an elongated tube with a lumen 16. Preferably, the proximal section 18 of the deployment catheter 12 is formed of a pellethane material having a durometer in a range of about 60 D to 75 D. The proximal section 18 is sufficiently flexible to transverse the vasculature of the human body, but is sufficiently rigid so that it can be pushed distally through the sheath 14. The distal section 20 of the deployment catheter 12 is preferably formed of a pellethane material having a durometer of between 25 D and 55 D with a durometer of 40 D being the preferred durometer.
[0029] The deployment catheter 12 also includes a winged hub 22 coupled to the proximal section 18 of the deployment catheter 12. The winged hub 22 may be made from plastic and aids in the insertion of the deployment catheter 12 into the vasculature of the bo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com