Antineoplastic combinations with mTOR inhibitor,herceptin, and/or hki-272
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Combination Regimen of Temsirolimus (CCI-779) and Herceptin in Trearment of Noeplasms
[0073] Dosing begins at month 1, day 1 with weekly intravenous (IV) temsirolimus and herceptin (IV) at the dosages provided below.
[0074] Temsirolimus and herceptin can be administered simultaneously, consecutively, or on alternative days.
[0075] Temsirolimus is administered IV weekly over a 30-minute period using an in-line filter and an automatic dispensing pump. Optionally, antihistamine (diphenhydramine, 25 to 50 mg IV or the equivalent) is administered about 30 minutes prior to temsirolimus infusion.
[0076] A herceptin loading dose is administered IV weekly over a 90 minute period. Weekly doses are administered, which are typically half the amount of the loading dose. For example, a 4 mg / kg loading dose is typically followed by 2 mg / kg weekly doses. These amounts may be adjusted. In one embodiment, no loading dose is required and the same dose is administered throughout the course of treatmen...
example 2
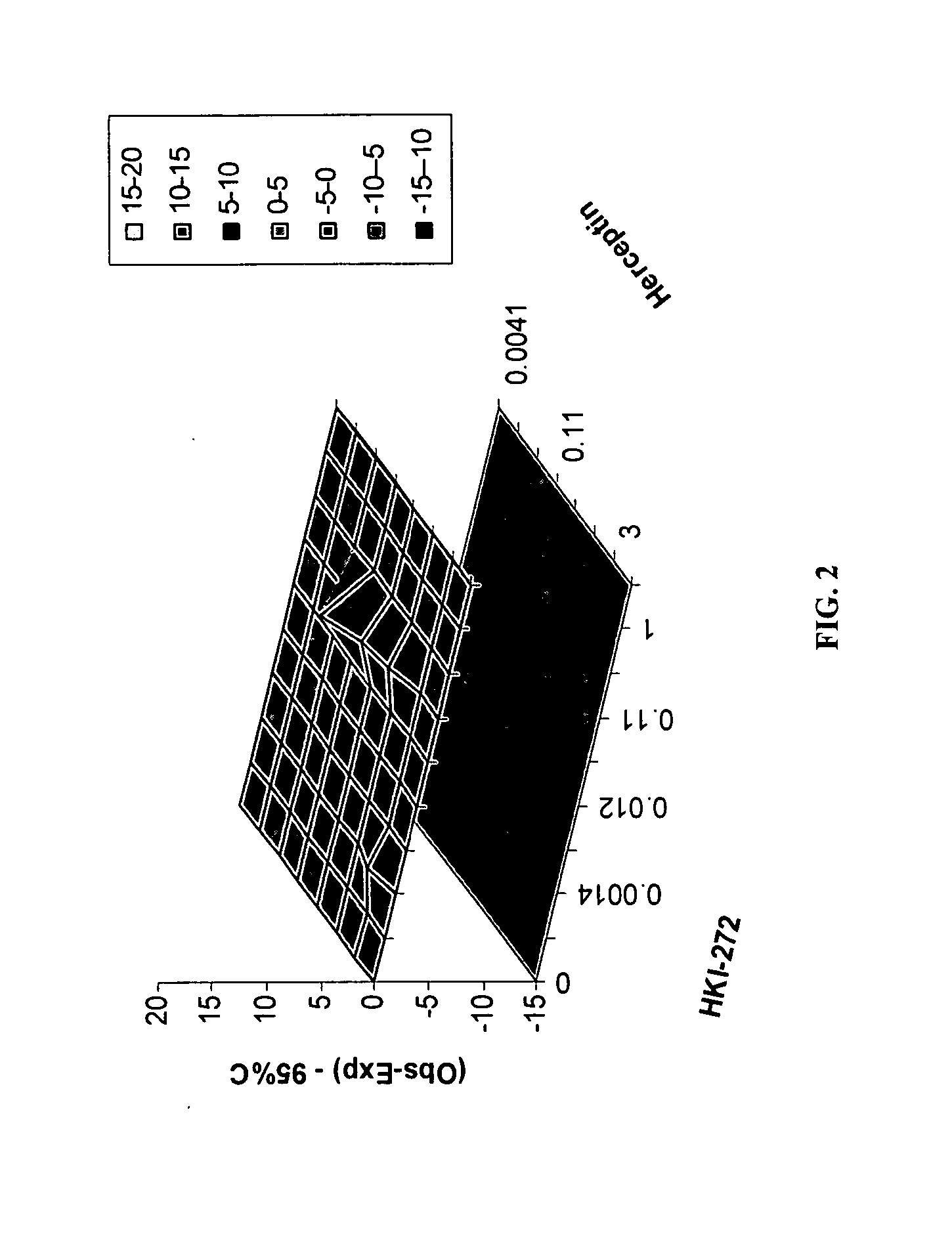
Use of a Combination Regimen of HKI-272 and Temsirolimus (CCI-779) in Treatment of Neoplasms
[0079] Dosing begins at month 1, day 1 with daily HKI-272 and weekly intravenous (IV) temsirolimus at the dosages provided below.
[0080] On month 1, day 1, HKI-272 is administered orally prior to temsirolimus. Temsirolimus is administered following HKI-272, preferably within 30 minutes.
[0081] Temsirolimus is administered IV weekly over a 30-minute period using an in-line filter and an automatic dispensing pump. Optionally, antihistamine (diphenhydramine, 25 to 50 mg IV or the equivalent) is administered about 30 minutes prior to temsirolimus infusion.
[0082] Thereafter, HKI-272 is taken orally once daily with food, preferably in the morning.
HKI-272Temsirolimus Dose(mg)(mg) 80151602524050
[0083] Dose adjustments and / or delays for HKI-272 and temsirolimus are permitted. For example, treatment may continue as described herein for six months, with daily doses of HKI-272 and weekly doses of te...
example 3
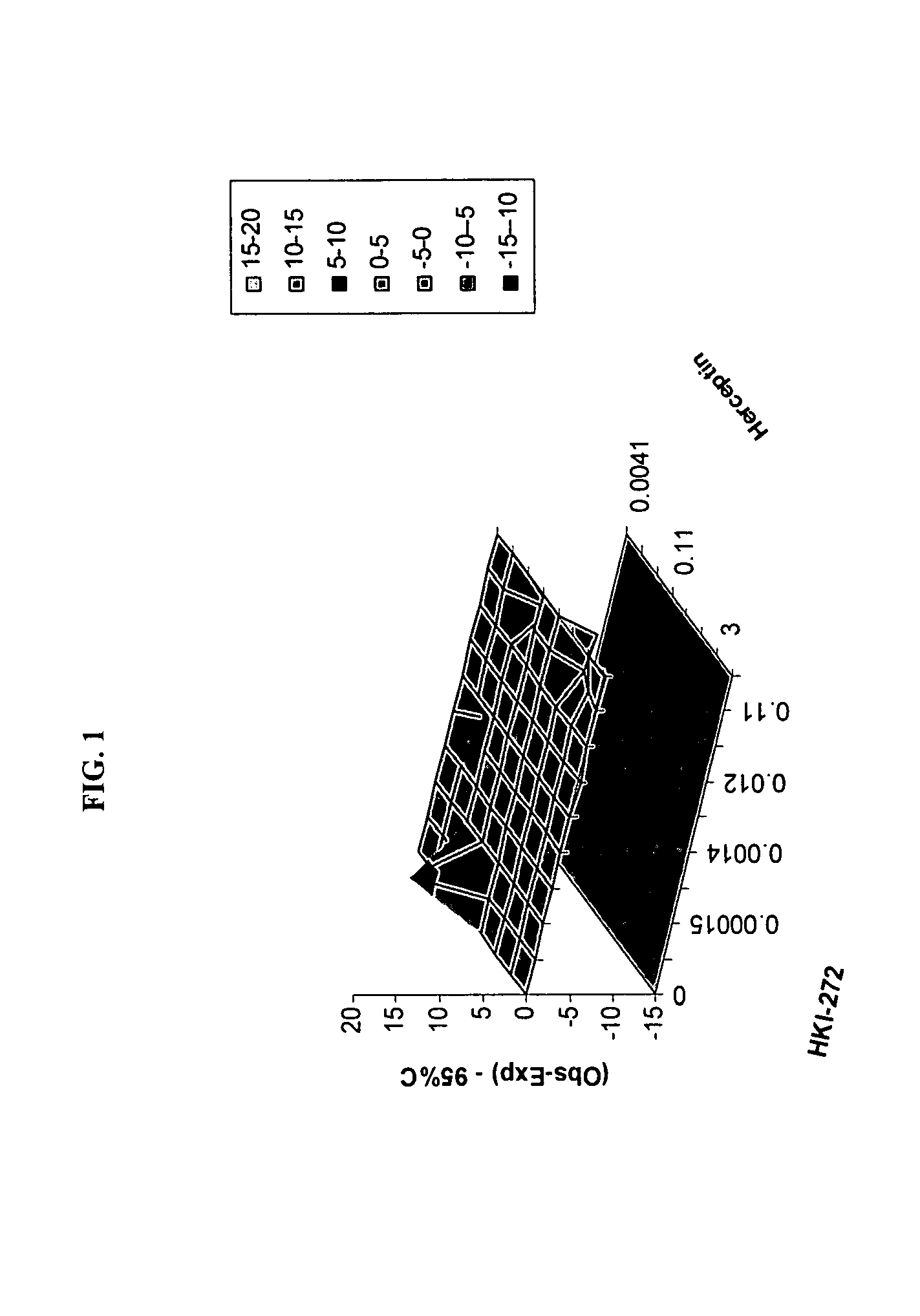
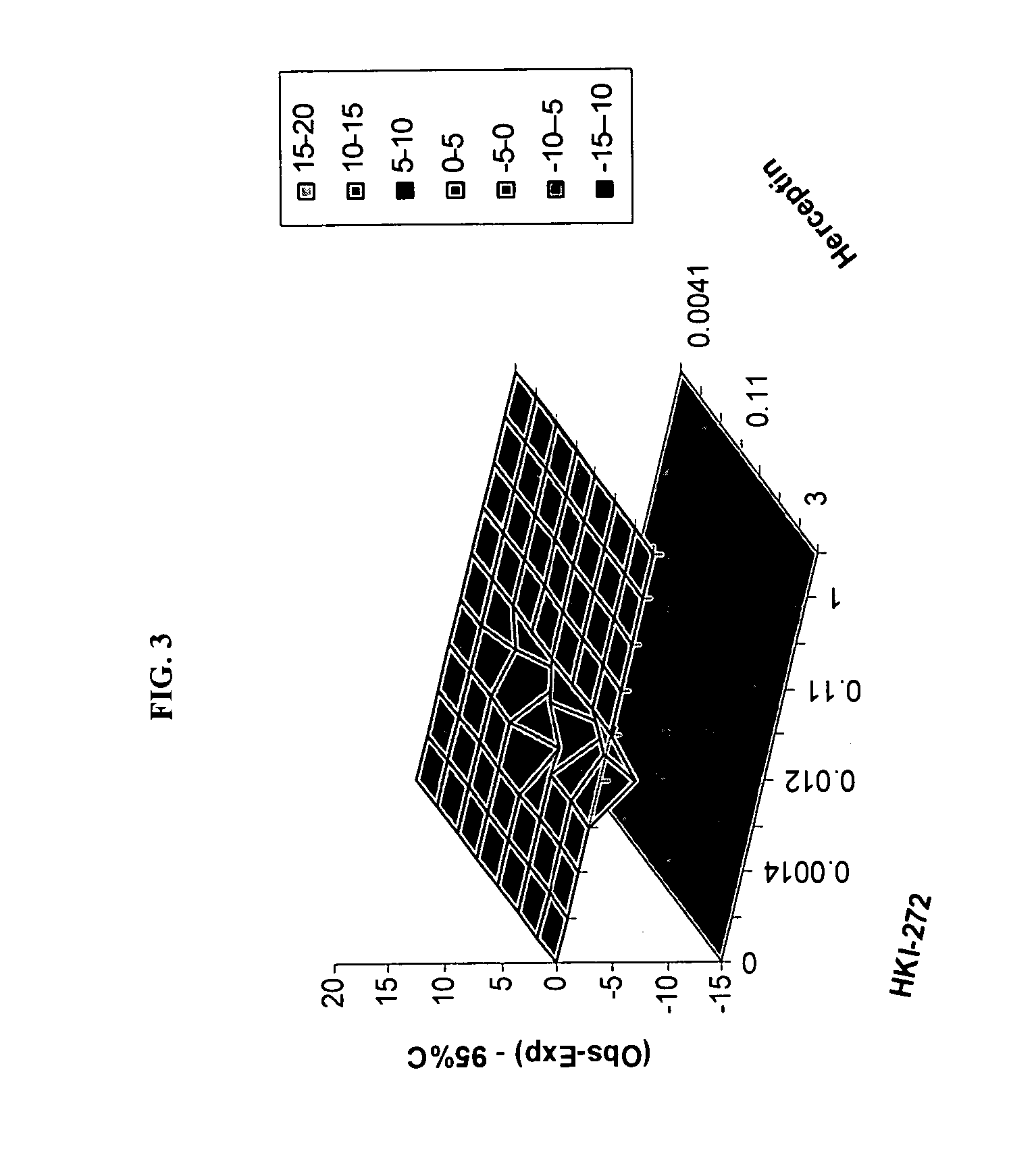
Use of a Combination Regimen of HKI-272, Temsirolimus (CCI-779), and Herceptin in Trearment of NEOPLASMS
[0084] Dosing begins at month 1, day1 with daily HKI-272 and weekly intravenous (IV) temsirolimus and herceptin (IV) at the dosages provided below.
[0085] On month 1, day 1, HKI-272 is administered orally prior to temsirolimus. Temsirolimus and herceptin are administered following HKI-272, preferably within 30 minutes.
[0086] Temsirolimus is administered IV weekly over a 30-minute period using an in-line filter and an automatic dispensing pump. Optionally, antihistamine (diphenhydramine, 25 to 50 mg IV or the equivalent) is administered about 30 minutes prior to temsirolimus infusion.
[0087] A herceptin loading dose is administered IV weekly over a 90 minute period. Weekly doses are administered, which are typically half the amount of the loading dose. For example, a 4 mg / kg loading dose is typically followed by 2 mg / kg weekly doses. These amounts may be adjusted. In one embodim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com