Limited Reuse Assembly For Ophthalmic Injection Device
a technology of ophthalmic injection device and assembly, which is applied in the direction of contraceptive device, eye treatment, surgery, etc., can solve the problems of affecting the accuracy of volume injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] Reference is now made in detail to the exemplary embodiments of the invention, examples of which are illustrated in the accompanying drawings. Wherever possible, the same reference numbers are used throughout the drawings to refer to the same or like parts.
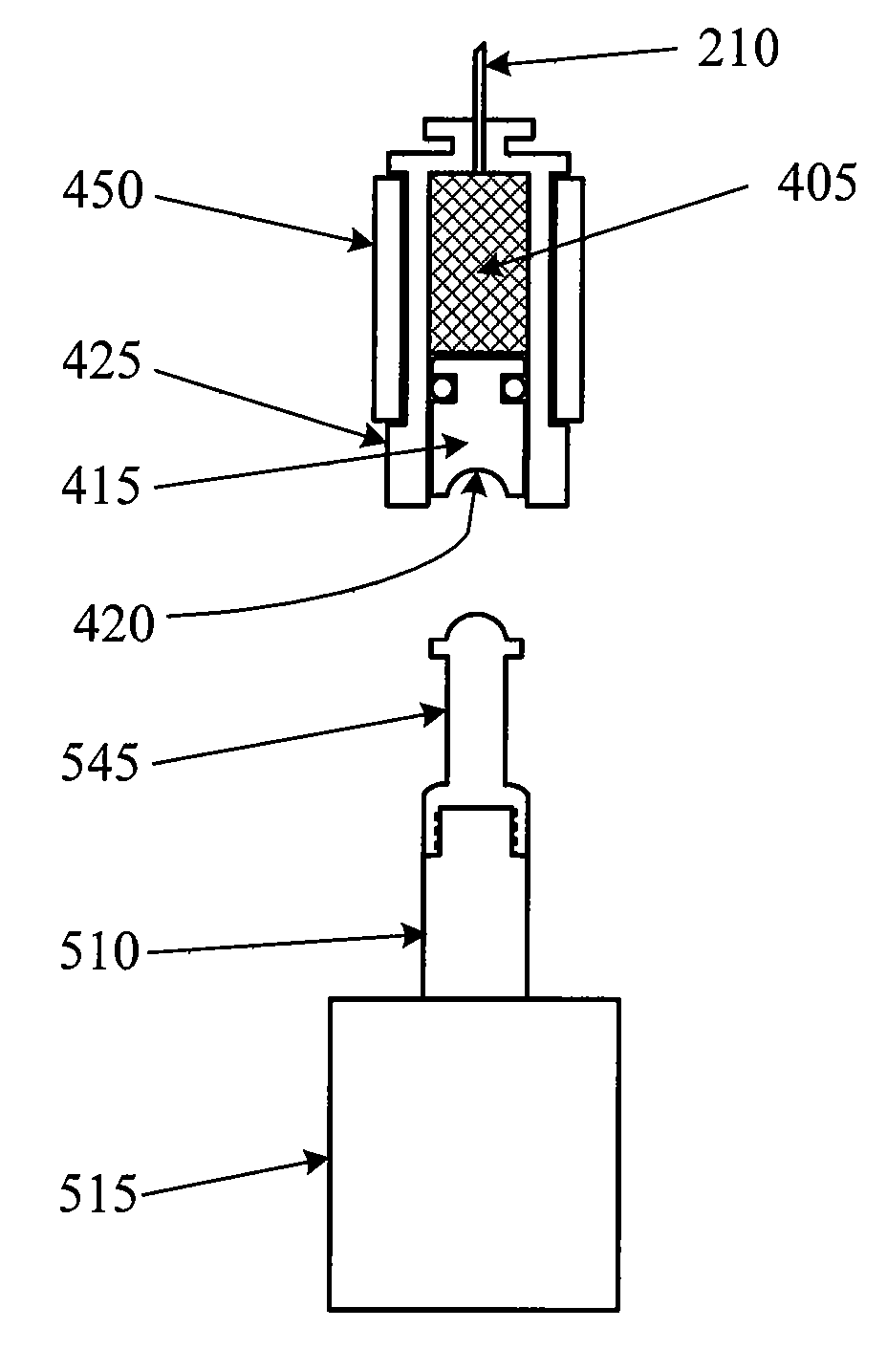
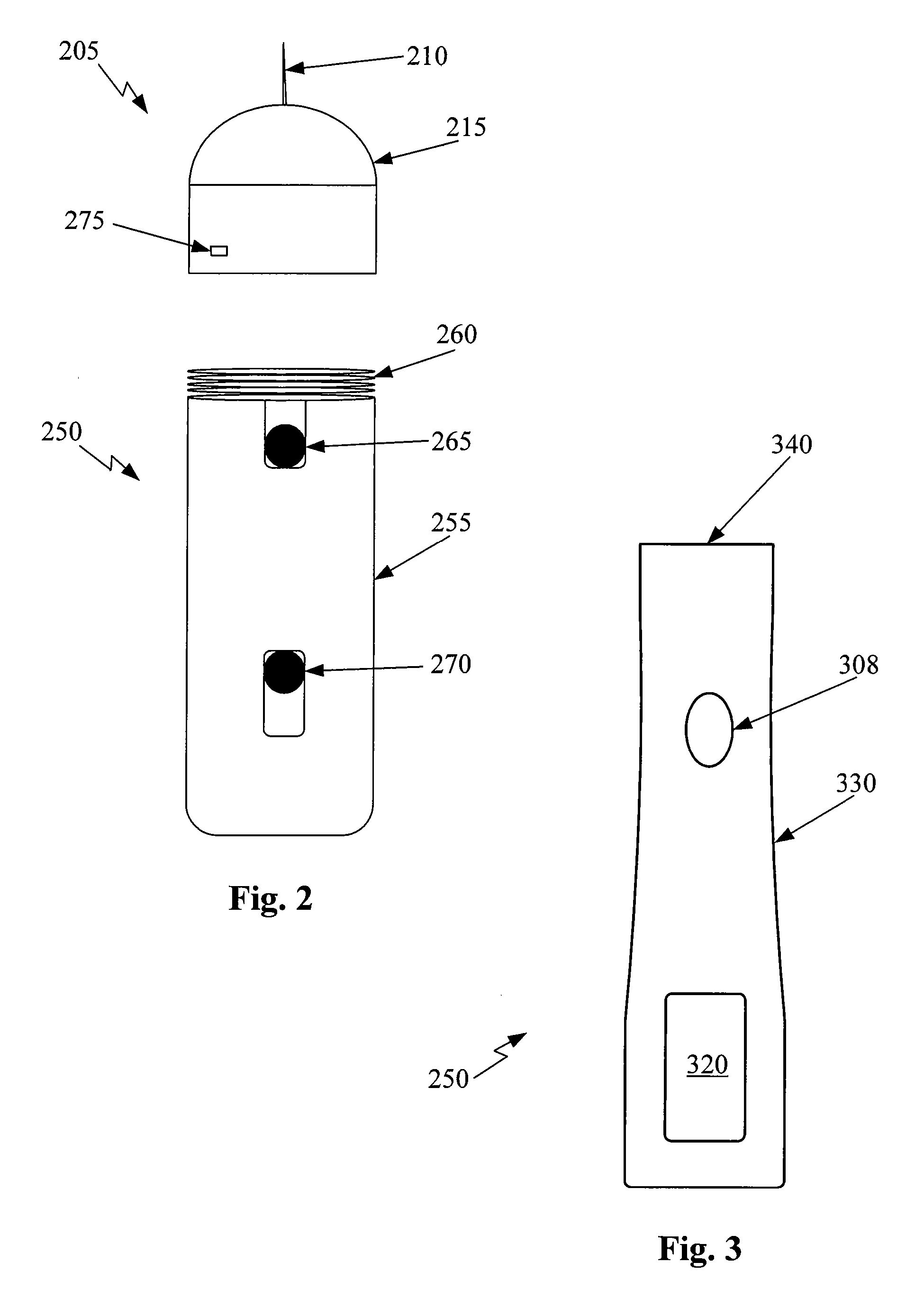
[0034]FIG. 2 is one view of an ophthalmic medical device including a disposable tip segment and a limited reuse assembly according to an embodiment of the present invention. In FIG. 2, the medical device includes a tip segment 205 and a limited reuse assembly 250. The tip segment 205 includes a needle 210, a housing 215, and an optional light 275. The limited reuse assembly 250 includes a housing 255, a switch 270, a lock mechanism 265, and a threaded portion 260.
[0035] Tip segment 205 is capable of being connected to and removed from limited reuse assembly 250. In this embodiment, tip segment 205 has a threaded portion on an interior surface of housing 215 that screws onto the threaded portion 260 of limited reuse assemb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com