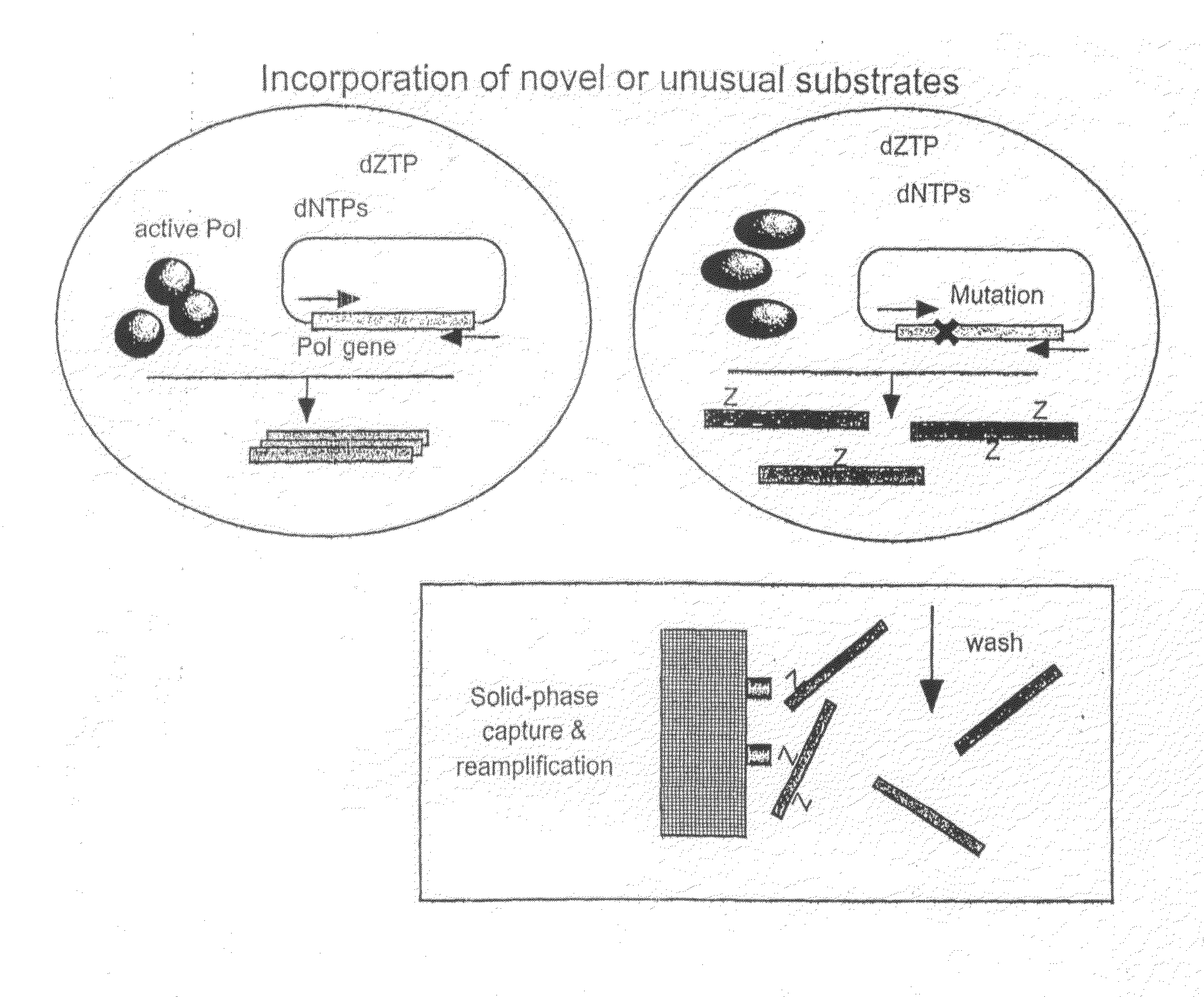
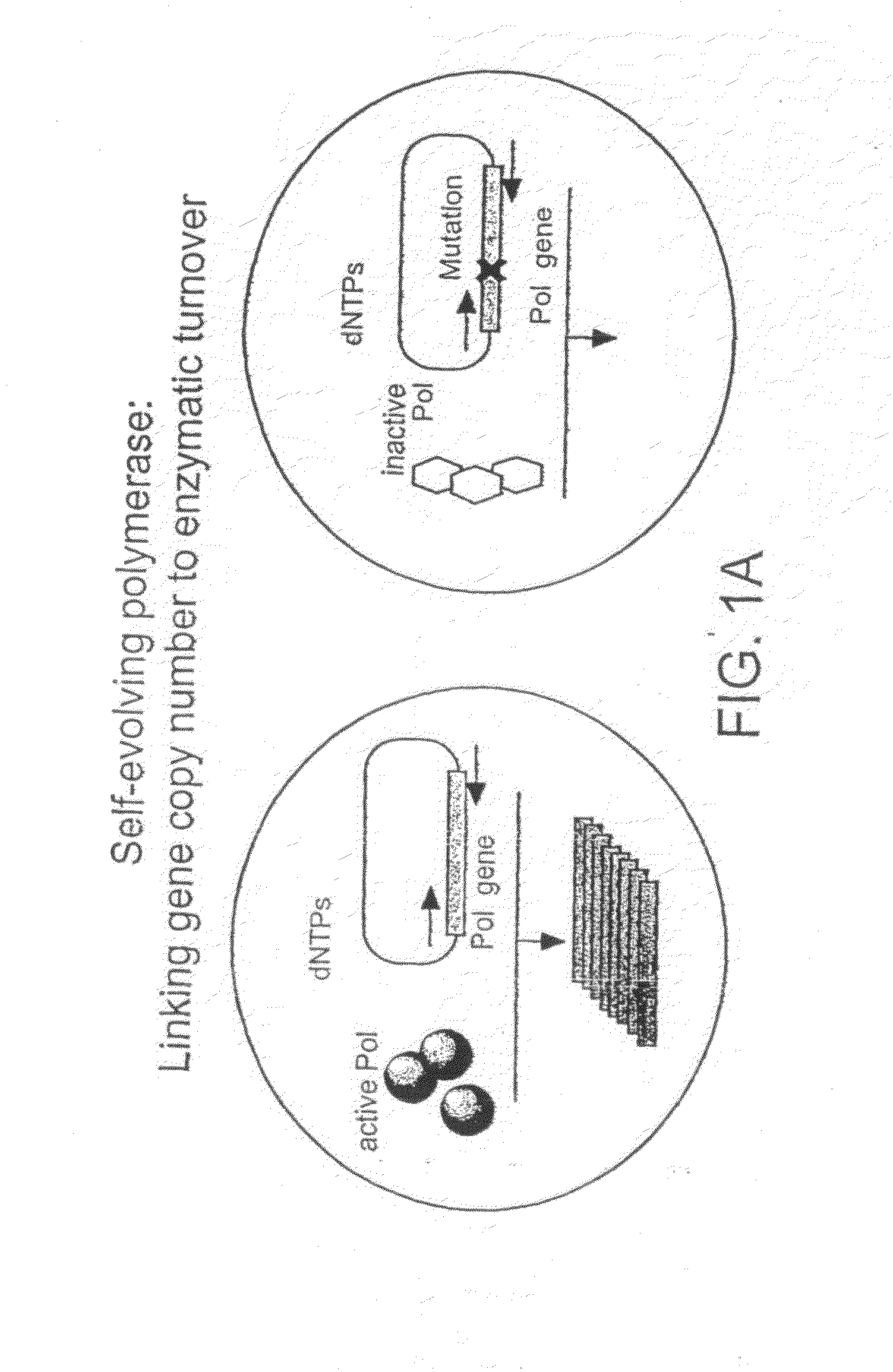
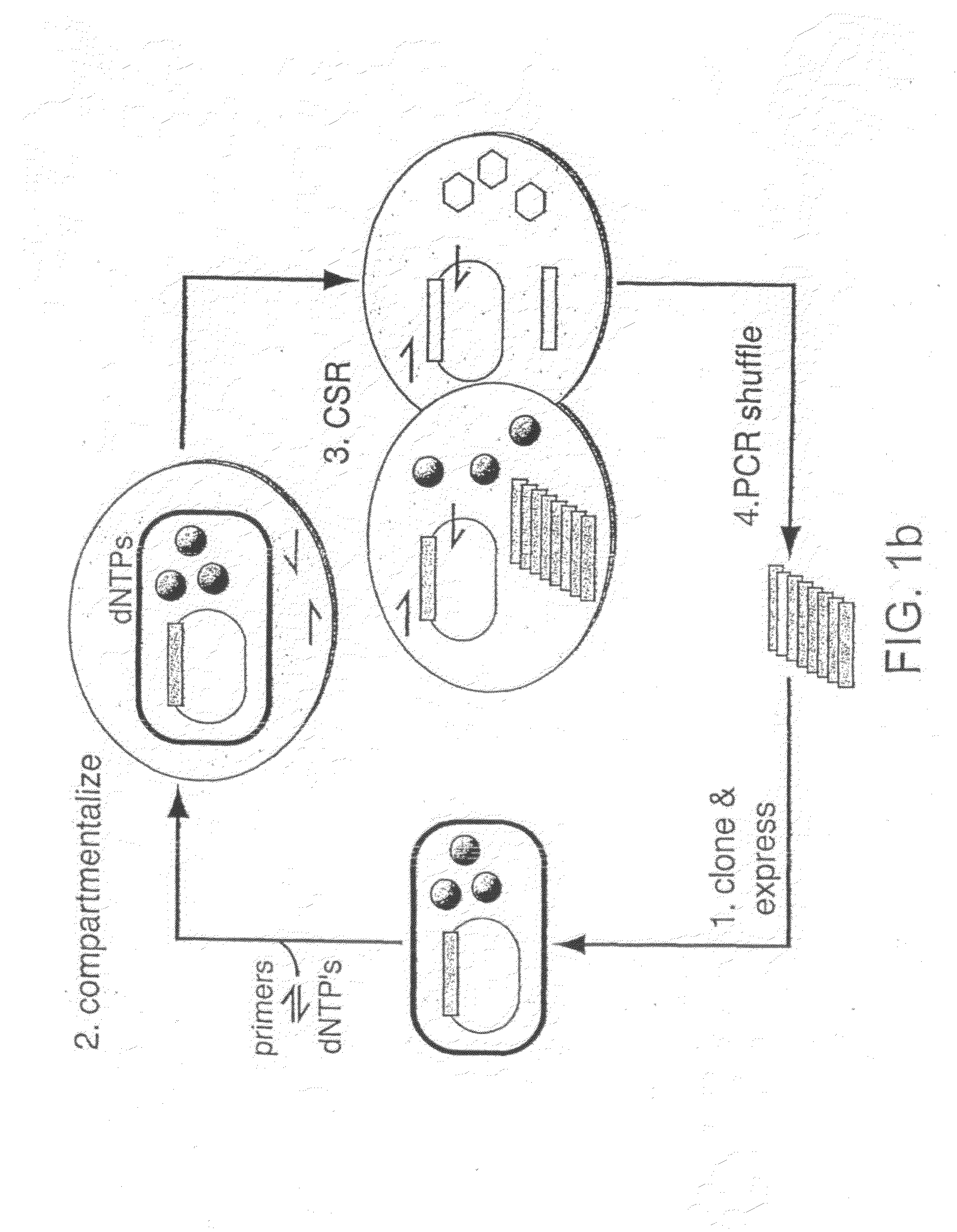
Methods of increasing the concentration of a nucleic acid
a nucleic acid and concentration technology, applied in the field of methods of increasing the concentration of nucleic acids, can solve the problems of difficult catalysis selection, serious shortcomings, and few attempts to improve the properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Taq Polymerase Expression Plasmids
[0239]The Taq polymerase open reading frame is amplified by PCR from Thermus aquaticus genomic DNA using primers 1 & 2, cut with XbaI & SalI and ligated into pASK75 (Skerra A., 1994, Gene 151, 131) cut with XbaI & SalI. pASK75 is an expression vector which directs the synthesis of foreign proteins in E. coli under transcriptional control of the tetA promoter / operator.
[0240]Clones are screened for inserts using primers 3, 4 and assayed for expression of active Taq polymerase (Taq pol) (see below). The inactive Taq pol mutant D785H / E786V is constructed using Quickchange mutagenesis (Stratagene). The mutated residues are critical for activity (Doublie S. et al., 1998, Nature 391, 251; Kiefer J. R. et al., 1998, Nature 391, 304). Resulting clones are screened for mutation using PCR screening with primers 3, 5 and diagnostic digestion of the products with PmlI. Mutant clones are assayed for expression of active Taq pol (see below).
example 2
Protein Expression and Activity Assay
[0241]Transformed TG1 cells are grown in 2×TY 0.1 mg / ml ampicillin. For expression, overnight cultures are diluted 1 / 100 into fresh 2×TY medium and grown to OD600=0.5 at 37° C. Protein expression is induced by addition of anhydro tetracycline to a final concentration of 0.2 μg / ml. After 4 hours further incubation at 37° C., cells are spun down, washed once, and re-suspended in an equal volume of 1× SuperTaq polymerase buffer (50 mM KCl, 10 mM Tris-HCl (pH9.0), 0.1% Triton X-100, 1.5 mM MgCl2) (HT Biotechnology Ltd, Cambridge UK).
[0242]Washed cells are added directly to a PCR reaction mix (2 μl per 30 μl reaction volume) comprising template plasmid (20 ng), primers 4 and 5 (1 μM each), dNTPs (0.25 mM), 1× SuperTaq polymerase buffer, and overlaid with mineral oil. Reactions are incubated for 10 min at 94° C. to release Taq pol from the cells and then thermocycled with 30 cycles of the profile 94° C. (1 min), 55° C. (1 min), 72° C. (2 min).
example 3
Emulsification of Amplification Reactions
[0243]Emulsification of reactions is carried out as follows. 200 μl of PCR reaction mix (Taq expression plasmid (200 ng), primers 3 and 4 (1 μM each), dNTPs (0.25 mM), Taq polymerase (10 units) is added dropwise (12 drops / min) to the oil phase (mineral oil (Sigma)) in the presence of 4.5% (v / v) Span 80 (Fluka), 0.4% (v / v) Tween 80 (Sigma) and 0.05% (v / v) Triton X100 (Sigma) under constant stirring (1000 rpm) in 2 ml round bottom biofreeze vials (Costar, Cambridge Mass.). After complete addition of the aqueous phase, stirring is continued for a further 4 minutes. Emulsified mixtures are then transferred to 0.5 ml thin-walled PCR tubes (100 μl / tube) and PCR carried out using 25 cycles of the profile 94° C. (1 min), 60° C. (1 min), 72° C. (3 min) after an initial 5 min incubation at 94° C. Reaction mixtures are recovered by the addition of a double volume of ether, vortexing and centrifugation for 2 minutes prior to removal of the ether phase. A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com