Heteropolyacid or heteropolyacid salt and microporous coordination polymer composite material and preparation method thereof
A technology of coordination polymers and heteropoly acid salts, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, chemical/physical processes, etc. Problems such as few practical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
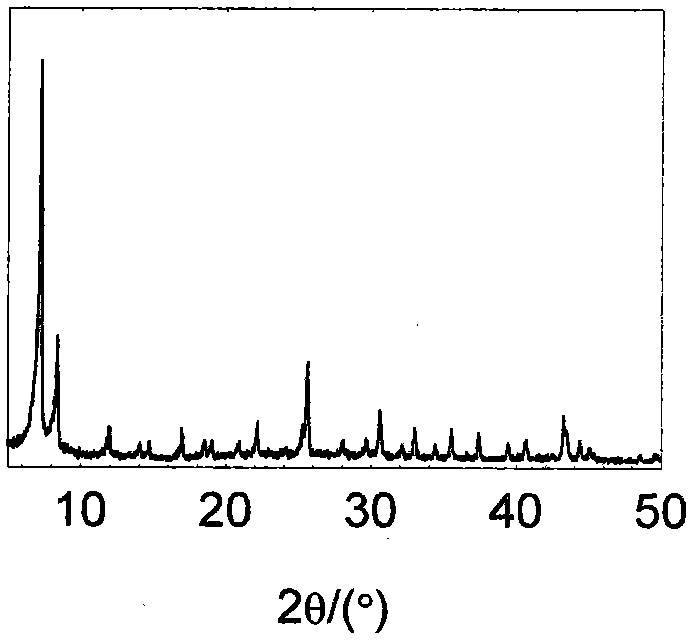
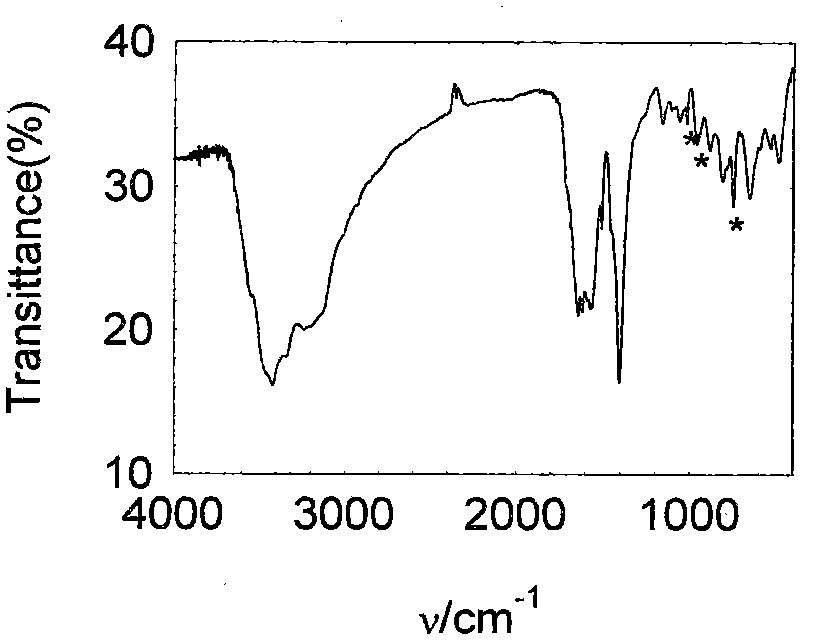
[0047] Dissolve 150 mg of zirconium tetrachloride (0.644 mmol) into a mixed solvent of 5 mL of acetic acid (87.4 mmol) and 50 mL of N,N'-dimethylformamide (645 mmol) under stirring to obtain a clear solution , after half an hour of continuous stirring, 130 mg of terephthalic acid (0.787 mmol) was dissolved in this mixture, followed by 12.2 mg of Na 3 PMo 12 o 40 ·xH 2 O (0.00644 mmol). The mixture was microwaved at 140°C for 2 hours, taken out, centrifuged, washed, and dried at 120°C to obtain the product with a yield of 91%. The product has been subjected to X-ray powder diffraction ( figure 1 ), its diffraction peak position is the same as that of UIO-66 (ZrO(O 2 C-C 6 h 4 -CO 2 )) are consistent, indicating that the main body of the composite material is UIO-66, and the crystallinity is relatively high; Fourier transform infrared spectroscopy analysis ( figure 2 ), the infrared characteristic vibration peak is consistent with UIO-66, and there is also the infrared...
Embodiment 2
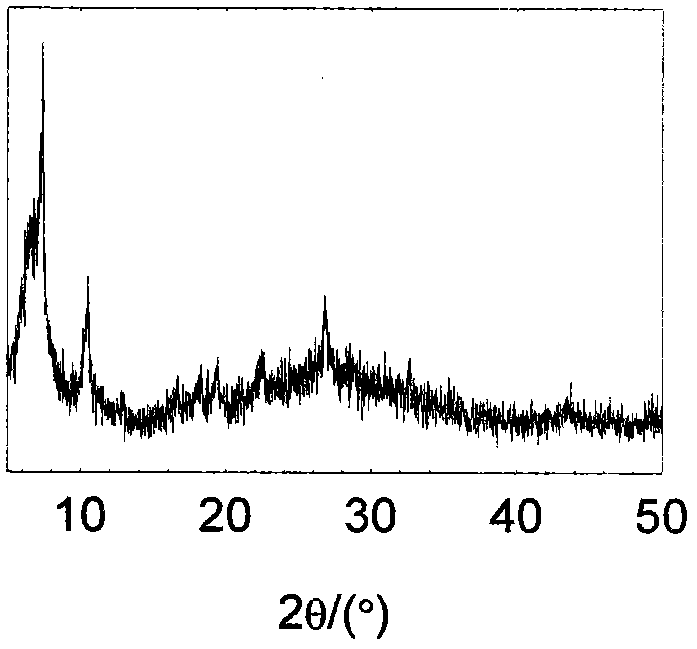
[0049] Dissolve 150 mg of zirconium tetrachloride (0.644 mmol) into a mixed solvent of 5 mL of acetic acid (87.4 mmol) and 40 mL of N,N'-dimethylformamide (516 mmol) under stirring to obtain a clear solution , after stirring continuously for half an hour, 156 mg of 2,6-naphthalene dicarboxylic acid (0.72 mmol) was dissolved in this mixture, followed by 167 mg of H 3 PMo 12 o 40 ·xH 2 O (0.092 mmol). The mixture was heated at 130°C for 36 hours, taken out, centrifuged, washed, and dried at 120°C to obtain the product with a yield of 89%. The product has been subjected to X-ray powder diffraction ( image 3 ), its diffraction peak position is the same as that of IRUIO-66 (ZrO(O 2 C-C 10 h 6 -CO 2 )) consistent, indicating that the main body of the composite material is IRUIO-66, and the crystallinity is relatively high; Fourier transform infrared spectroscopy analysis ( Figure 4 ), the infrared characteristic vibration peak is consistent with IRUIO-66, and there is als...
Embodiment 3
[0051] 131 mg of zirconyl sulfate (0.644 mmol) was dissolved under stirring into a mixed solvent of 3 ml of acetic acid (52.4 mmol) and 40 ml of N, N'-dimethylformamide (516 mmol) to obtain a clear solution, After continuous stirring for half an hour, 125 mg of 4,4'-biphenyldicarboxylic acid (0.51 mmol) was dissolved in this mixture, followed by 59 mg of H 3 PMo 12 o 40 ·xH 2 O (0.0322 mmol). The mixture was heated at 130°C for 36 hours, taken out, centrifuged, washed, and dried at 120°C to obtain the product with a yield of 85%. The product has been subjected to X-ray powder diffraction ( Figure 5 ), its diffraction peak position is the same as that of UIO-67 (ZrO(O 2 C-C 12 h 8 -CO 2 )) consistent, indicating that the main body of the composite material is UIO-67, and the crystallinity is relatively high; Fourier transform infrared spectroscopy analysis ( Figure 6 ), the infrared characteristic vibration peak is consistent with UIO-67, and there is also the infrar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity at room temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com