O-methylated rapamycin derivatives for alleviation and inhibition of lymphoproliferative disorders
a technology of rapamycin and derivatives, which is applied in the field of omethylated rapamycin derivatives for alleviating and inhibiting lymphoproliferative disorders, can solve the problems of malignant lymphoma type ptlds, the ineffective modification of conventional treatment with conventional agents, and the ability of the body, so as to alleviate the disorder, alleviate the disorder, and alleviate the effect of lymphoproliferative disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
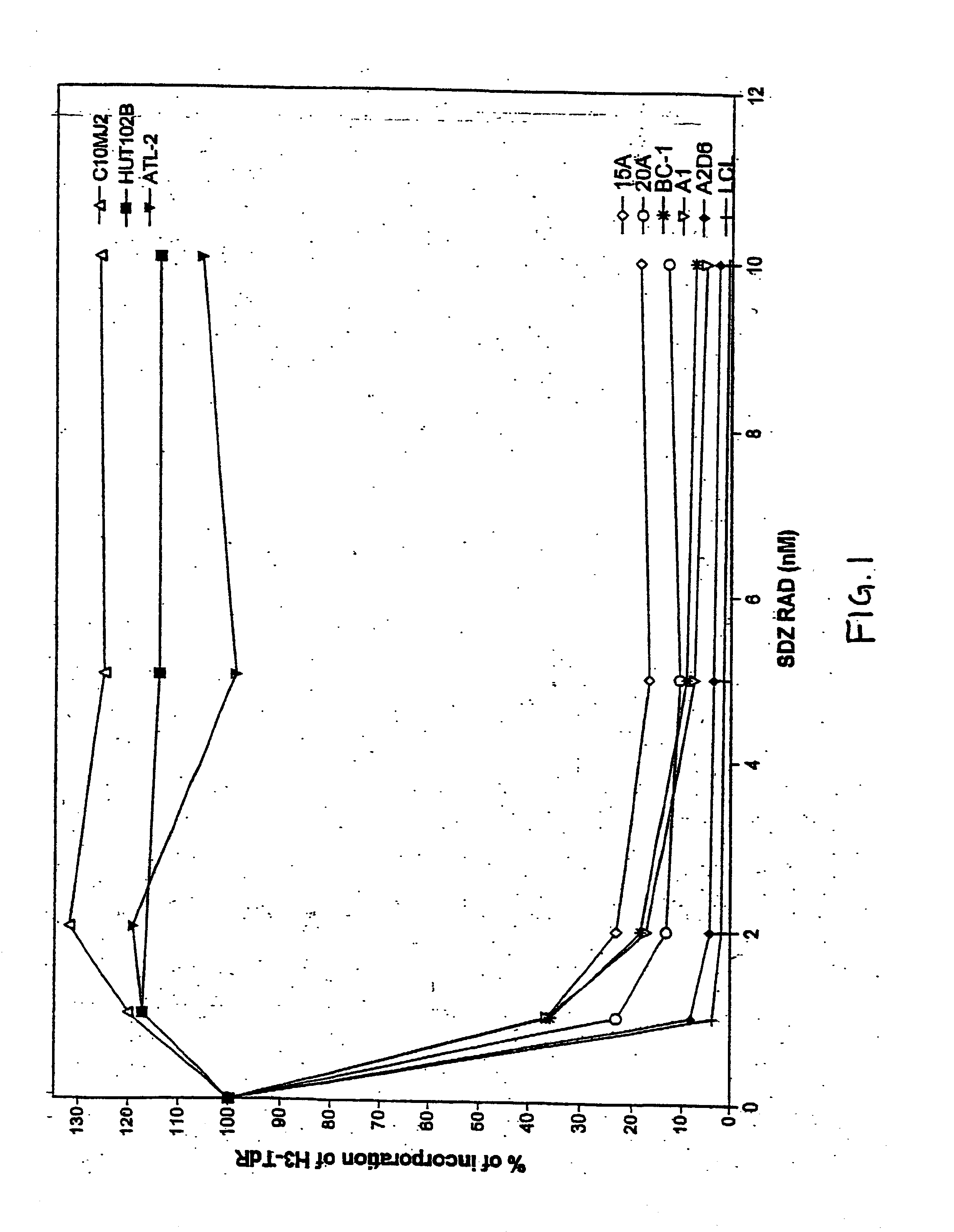
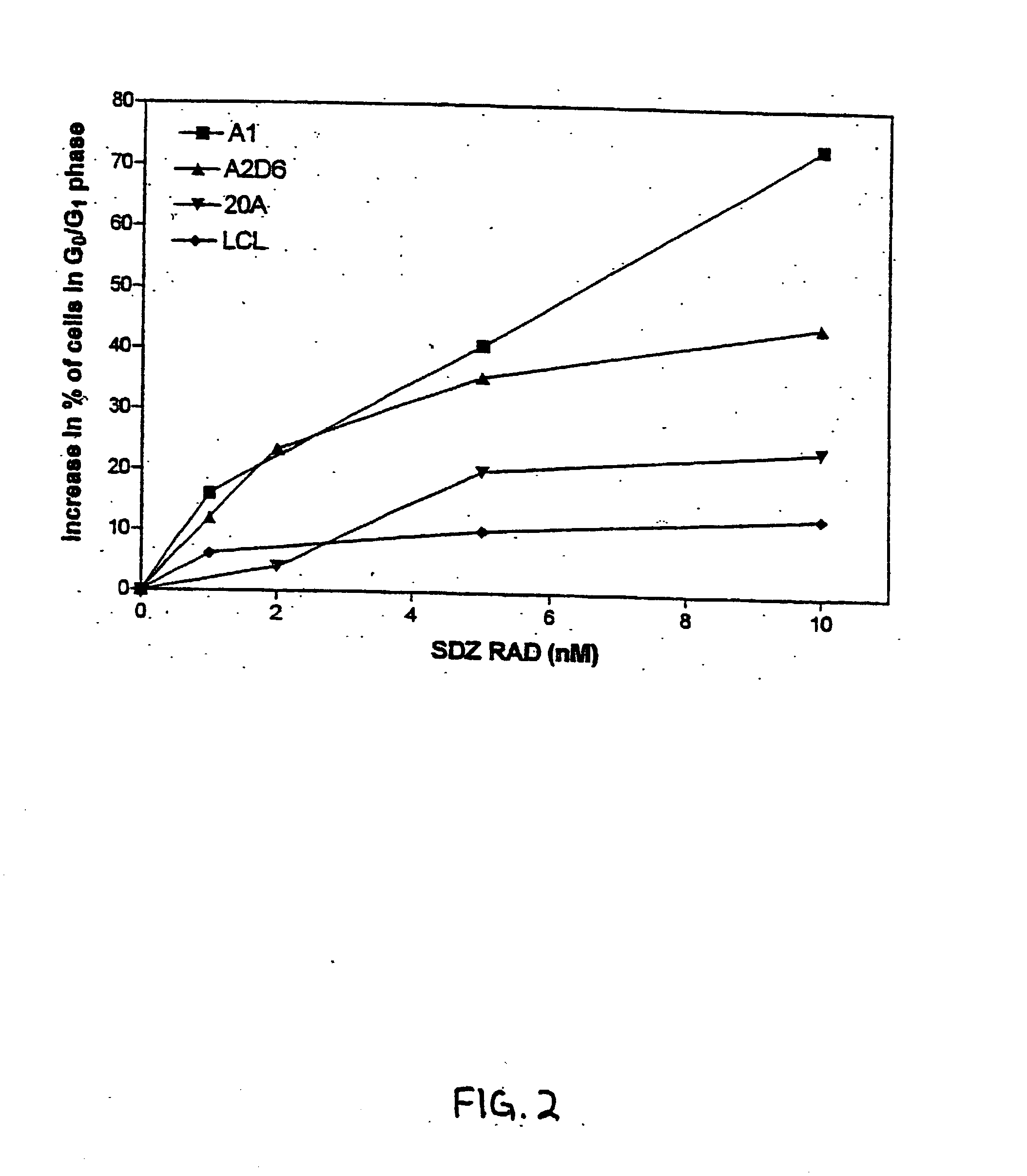
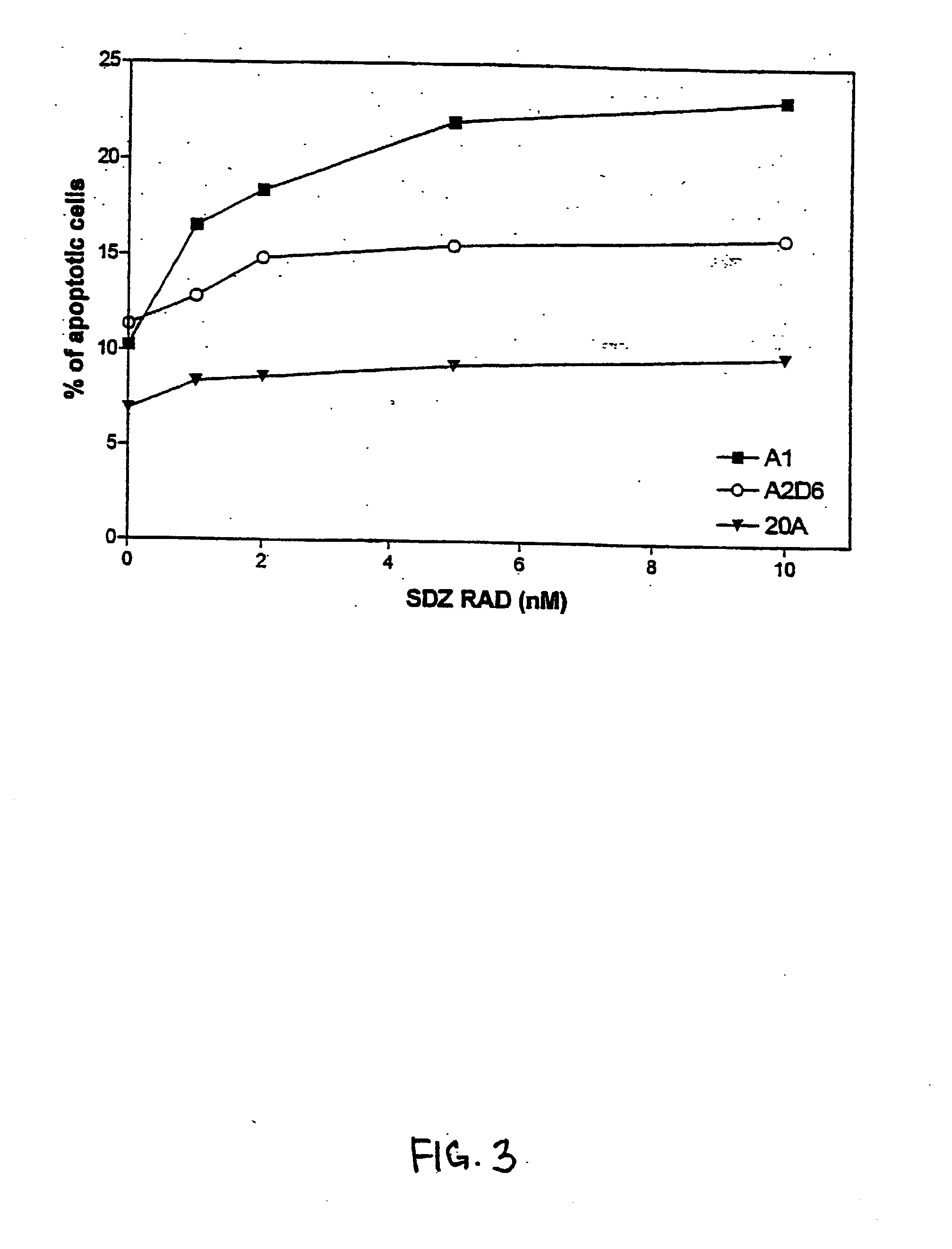
SDZ RAD Inhibits Growth of Human EBV-Transformed B Lymphocytes In Vitro and In Vivo
[0101] The experiments presented in this Example demonstrate that SDZ RAD is a potent anti-rejection, anti-lymphoproliferative agent, and represents a novel approach to inhibition and treatment of post-transplant lymphoproliferative disorders (PTLDs).
[0102] The materials and methods used in the experiments presented in this Example are now described.
[0103] Most cell lines used in this study were lymphoblastoid B-cell lines obtained by in vitro infection with EBV of peripheral blood mononuclear cells (PBMC). Cell lines A1 and A2D6 were obtained from normal, healthy individuals. Cell lines 15A and 20A were obtained from two different patients with a low grade B-cell lymphoma with monoclonal cold agglutinins (Silberstein et al., 1991, Blood 78:2372-2386). Both lines secreted cold agglutinins with the same specificity as the cold agglutinins found in the patient's serum (Silberstein et al., 1991, Blood...
PUM
| Property | Measurement | Unit |
|---|---|---|
| w/w | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com