Thin Film Devices for Temporary or Permanent Occlusion of a Vessel
a thin film, temporary or permanent technology, applied in the field of medical devices, can solve the problems of inaccurate occlusion, extremely small pores, time-consuming and complicated methods, etc., and achieve the effect of reducing cross-sectional extent and facilitating endoluminal deploymen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028]As required, detailed embodiments of the present invention are disclosed herein; however, it is to be understood that the disclosed embodiments are merely exemplary of the invention, which may be embodied in various forms. Therefore, specific details disclosed herein are not to be interpreted as limiting, but merely as a basis for the claims and as a representative basis for teaching one skilled in the art to variously employ the present invention in virtually any appropriate manner.
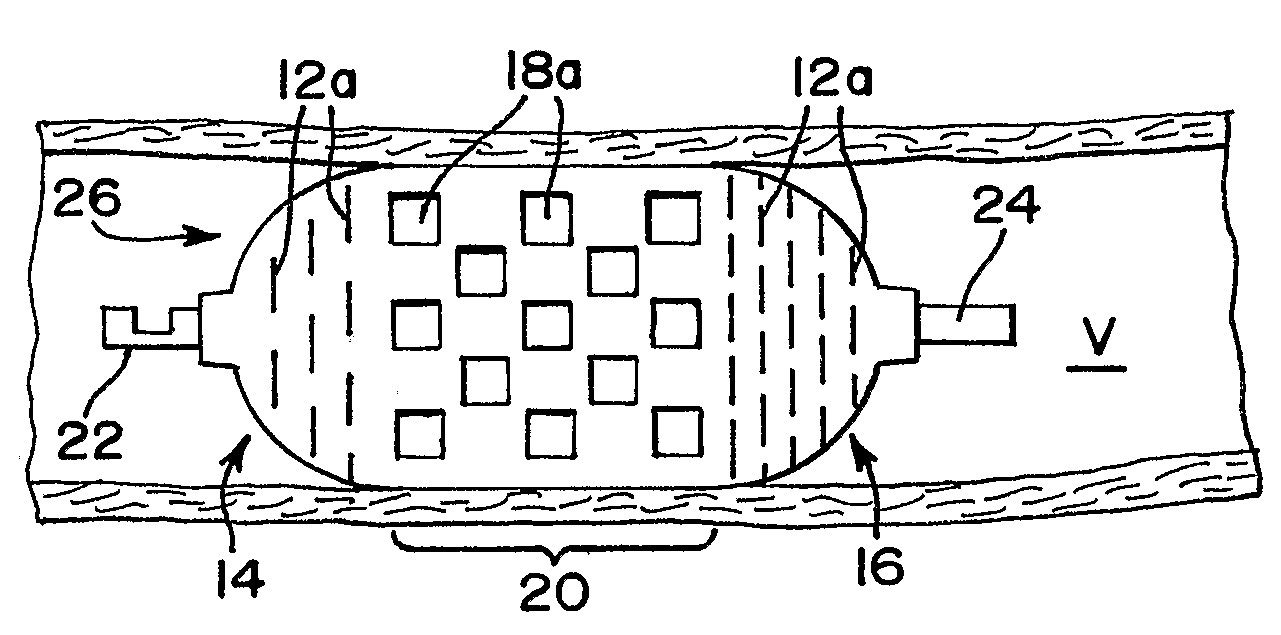
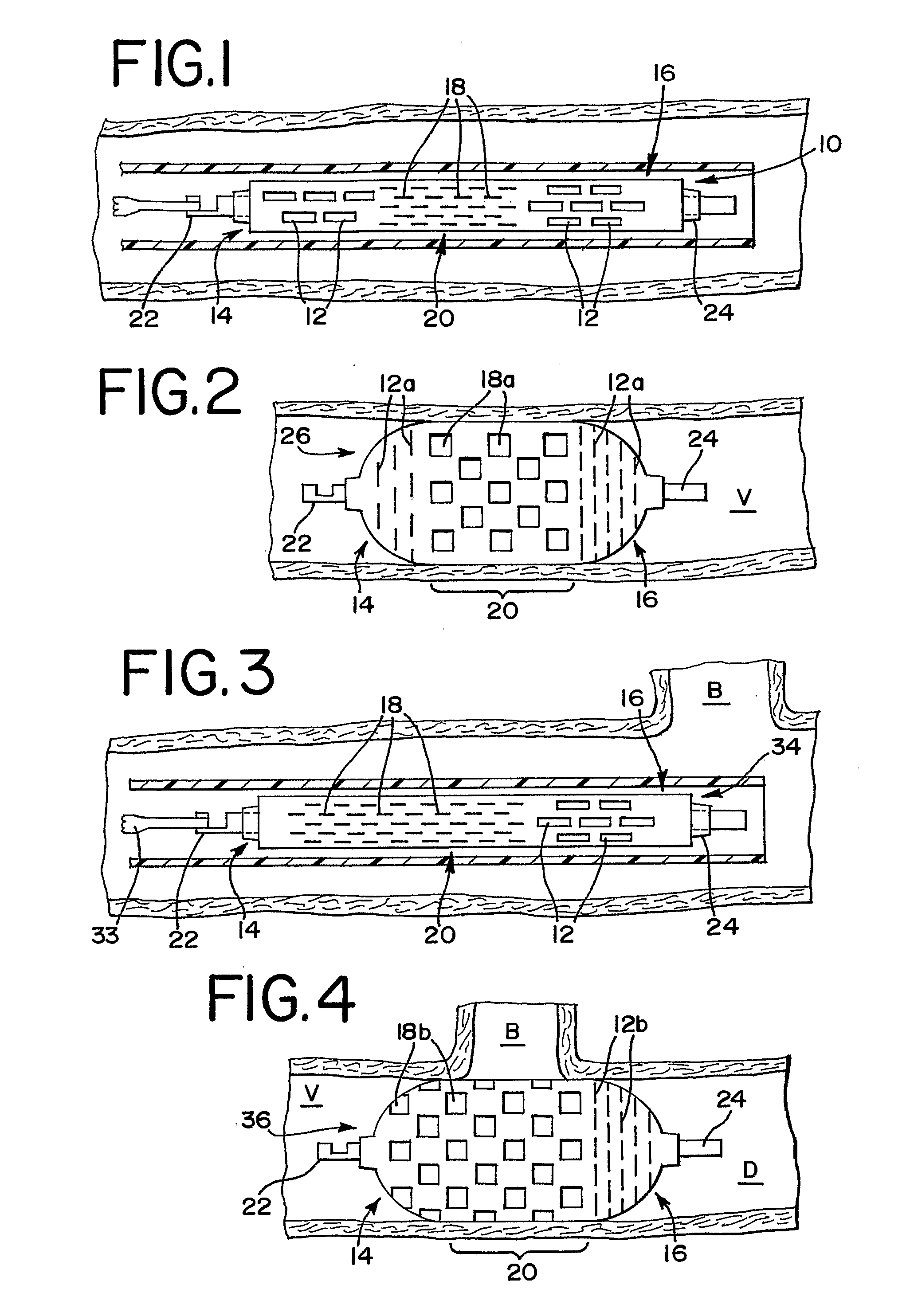
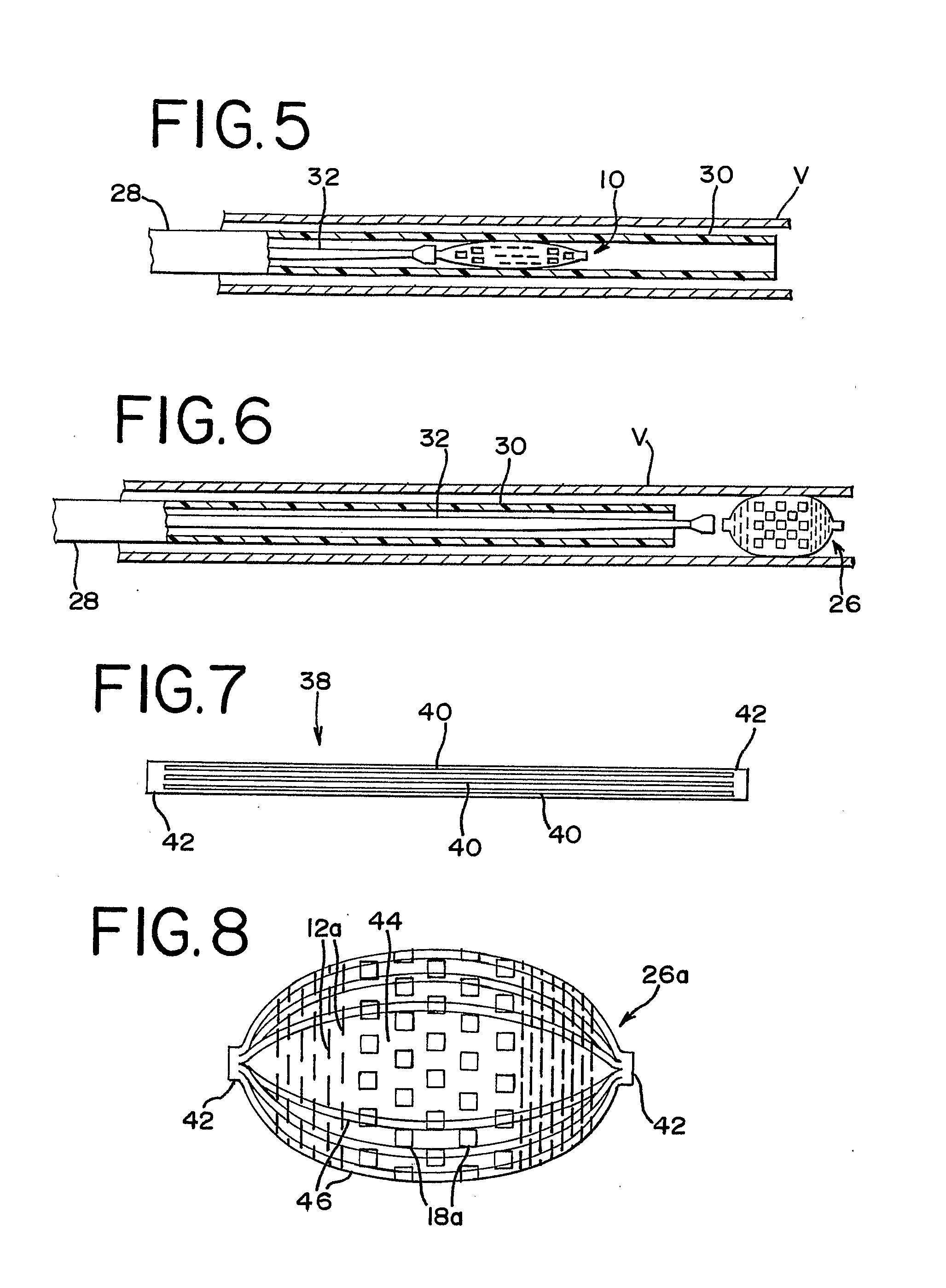
[0029]FIG. 1 illustrates an occlusion device 10 in a collapsed position. The occlusion device 10 preferably comprises a thin film mesh formed by physical vapor deposition onto a core or mandrel, as is well-known to those skilled in the art. Most preferably, a thin film of nitinol, or other material which preferably has the ability to take on a shape that had been imparted to it during manufacture, is formed. When nitinol material is used in forming the thin film, the thin film can be at the martens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com