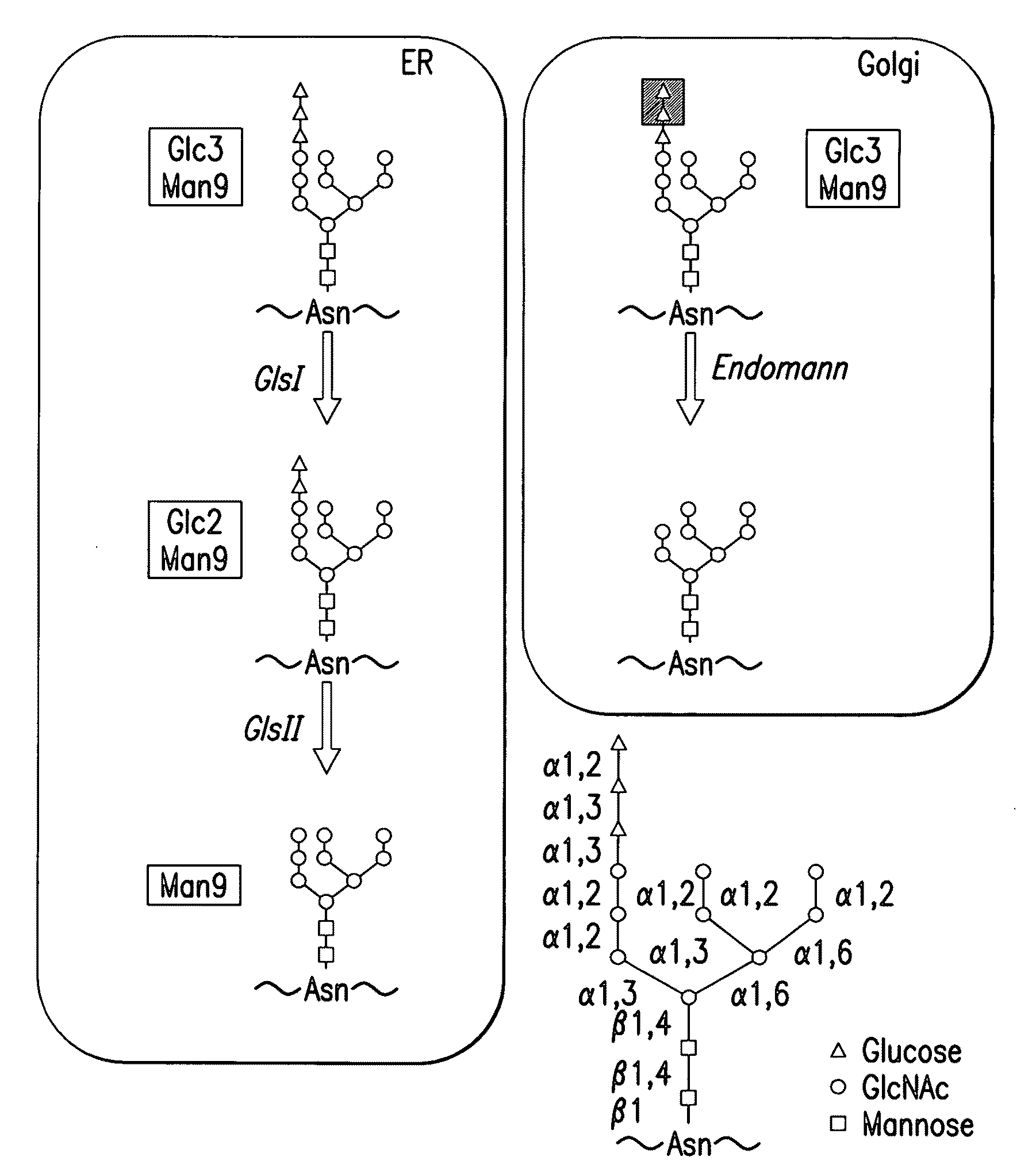
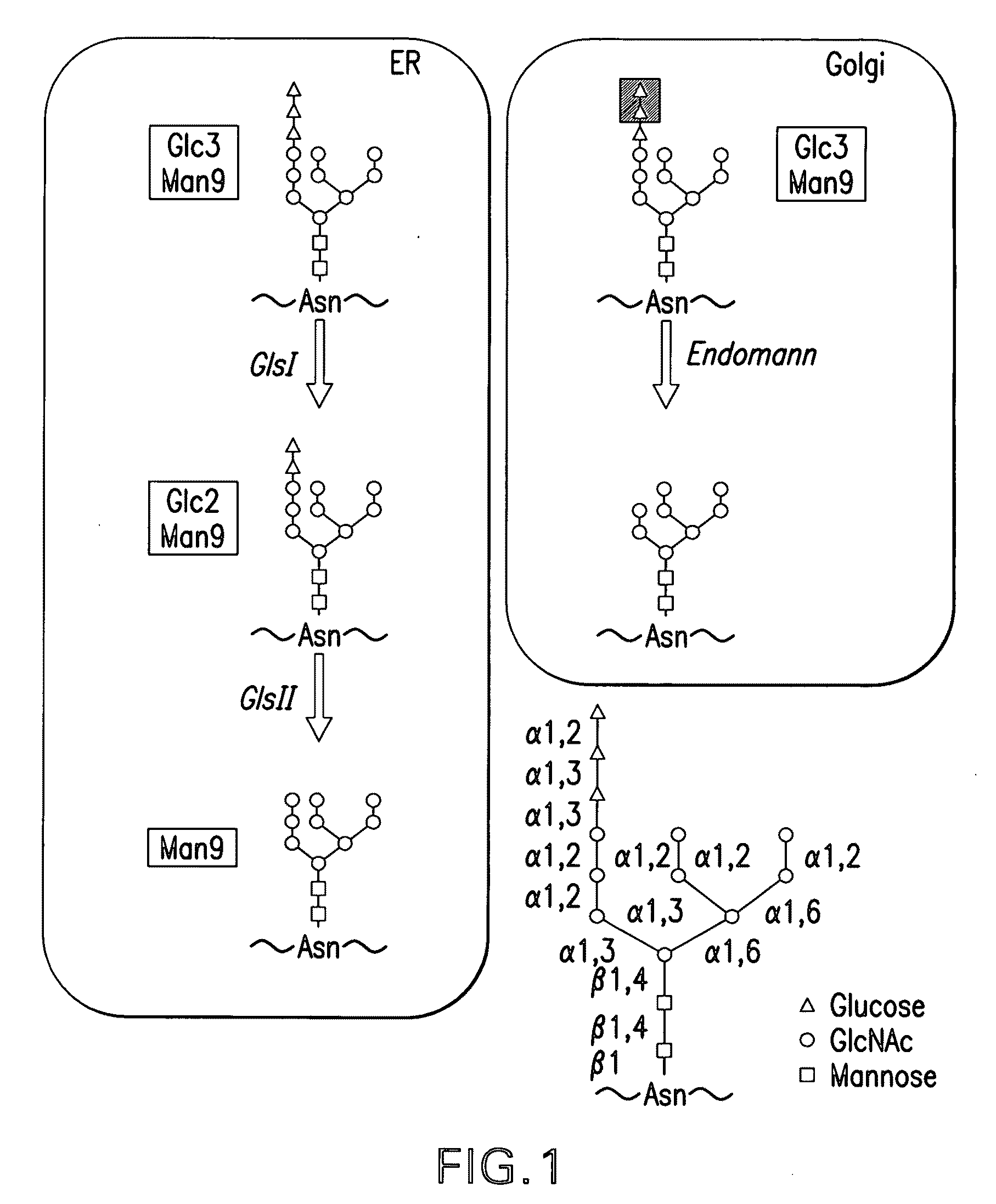
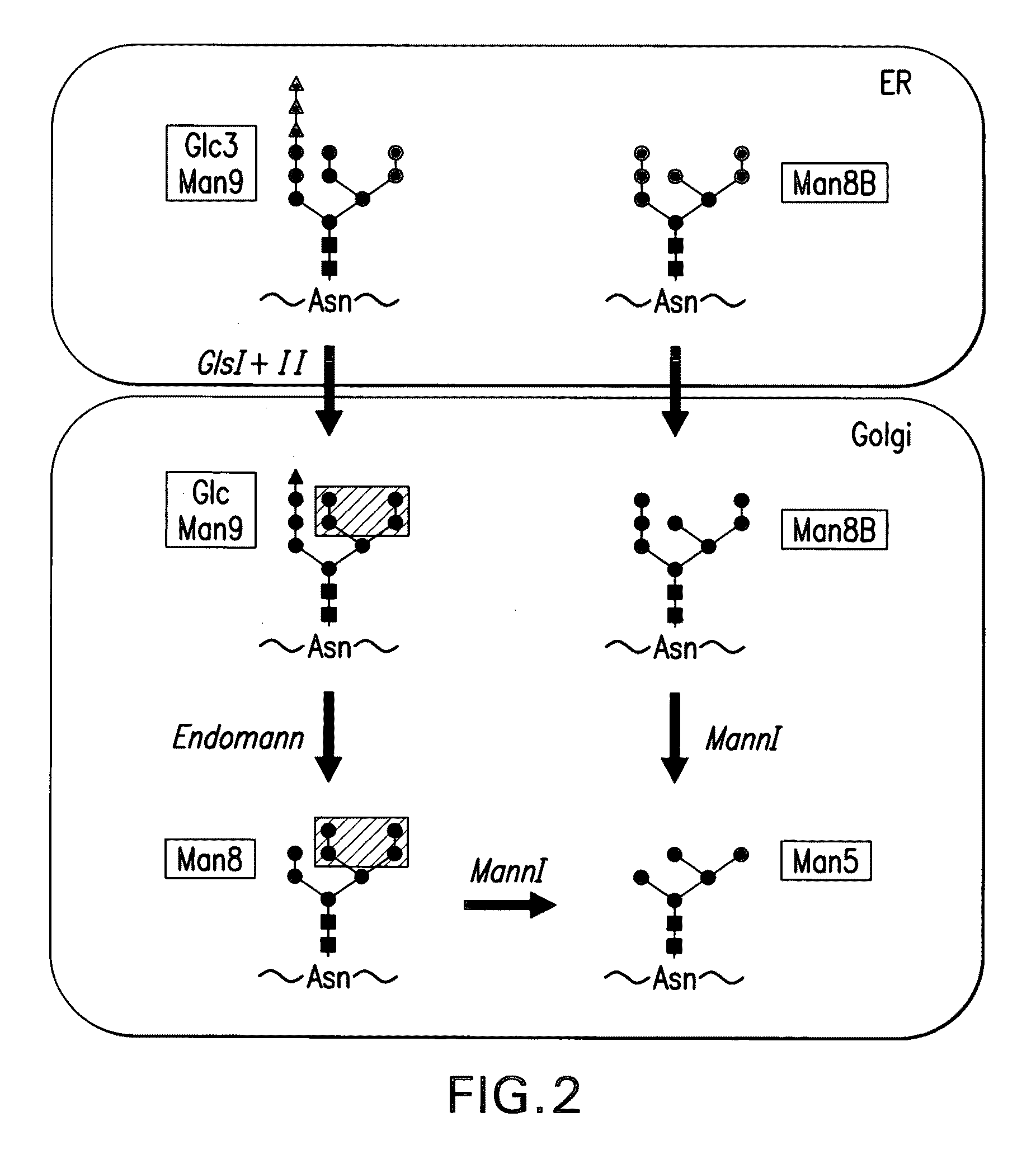
Endomannosidases in the modification of glycoproteins in eukaryotes
a technology of endomannosidases and eukaryotes, applied in the field of endomannosidases in the modification of glycoproteins in eukaryotes, can solve the problems of slow inherent problems of all mammalian expression systems, etc., and achieve the effect of improving the processing of n-linked glycan structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Strains, Culture Conditions, and Reagents
[0155]Escherichia coli strains TOP10 or DH5a were used for recombinant DNA work. Protein expression in yeast strains were carried out at room temperature in a 96-well plate format with buffered glycerol-complex medium (BMGY) consisting of 1% yeast extract, 2% peptone, 100 mM potassium phosphate buffer, pH 6.0, 1.34% yeast nitrogen base, 4×10−5% biotin, and 1% glycerol as a growth medium. The induction medium was buffered methanol-complex medium (BMMY) consisting of 1.5% methanol instead of glycerol in BMGY. Minimal medium is 1.4% yeast nitrogen base, 2% dextrose, 1.5% agar and 4×10−5% biotin and amino acids supplemented as appropriate. Restriction and modification enzymes were from New England BioLabs (Beverly, Mass.). Oligonucleotides were obtained from the Dartmouth College Core facility (Hanover, N.H.) or Integrated DNA Technologies (Coralville, Iowa). MOPS, sodium cacodylate, manganese chloride were from Sigma (St. Louis, Mo.). Trifluoroa...
example 2
Cloning of Human and Mouse Endomannosidases
[0156]As a positive control, we amplified the region homologous to the putative catalytic domain of the rat mannosidase gene using specific primers 5′-gaattcgccaccatggatttccaaaagagtgacagaatcaacag-3′ (SEQ ID NO: 11) and 5′-gaattcccagaaacaggcagctggcgatc-3′ (SEQ ID NO: 12) and subcloned the resultant region into a yeast integration plasmid using standard recombinant DNA techniques (See, e.g., Sambrook et al. (1989) Molecular Cloning, A Laboratory Manual (2nd ed.), Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. and references cited therein, all incorporated reference; see also Example 3).
[0157]To identify the sequence of and isolate the ORF of the human endomannosidase, we performed a protein BLAST search using the rat endomannosidase protein sequence (Genbank gi:2642187) and identified a hypothetical human protein (Genbank gi:20547442) of 290 amino acids in length which shows 88% identity and 94% similarity to amino acids 162 to 451 o...
example 3
Generation of Recombinant Endomannosidase Constructs and Expression
[0160]To generate a yeast secreted form of the human endomannosidase, a region encoding the putative catalytic domain was expressed in the EasySelect Pichia Expression kit (Invitrogen) as recommended by the manufacturer. Briefly, PCR was used to amplify the ORF fragment from 178 to 1386 bases from pSH131 using the primers hEndo Δ59 forward and hEndo Δstop reverse (5′-gaattcgccaccatggatttccaaaagagtgacagaatcaacag-3′ (SEQ ID NO: 11) and 5′-gaattcccagaaacaggcagctggcgatc-3′ (SEQ ID NO: 12), respectively, with an EcoRI restriction site engineered into each). The conditions used with Pfu Turbo were: 95° C. for 1 min, 1 cycle; 95° C. for 30 sec, 55° C. for 30 sec, 72° C. for 3 min, 25 cycles; 72° C. for 3 min, 1 cycle. The product was incubated with Taq DNA polymerase, TOPO cloned and ABI sequenced as described above. The resulting clone was designated pSH178. From this construct, the human endomannosidase fragment was excis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com