Use of amidoxime carboxylic acid esters and n-hydroxyguanidine carboxylic acid esters for producing prodrugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
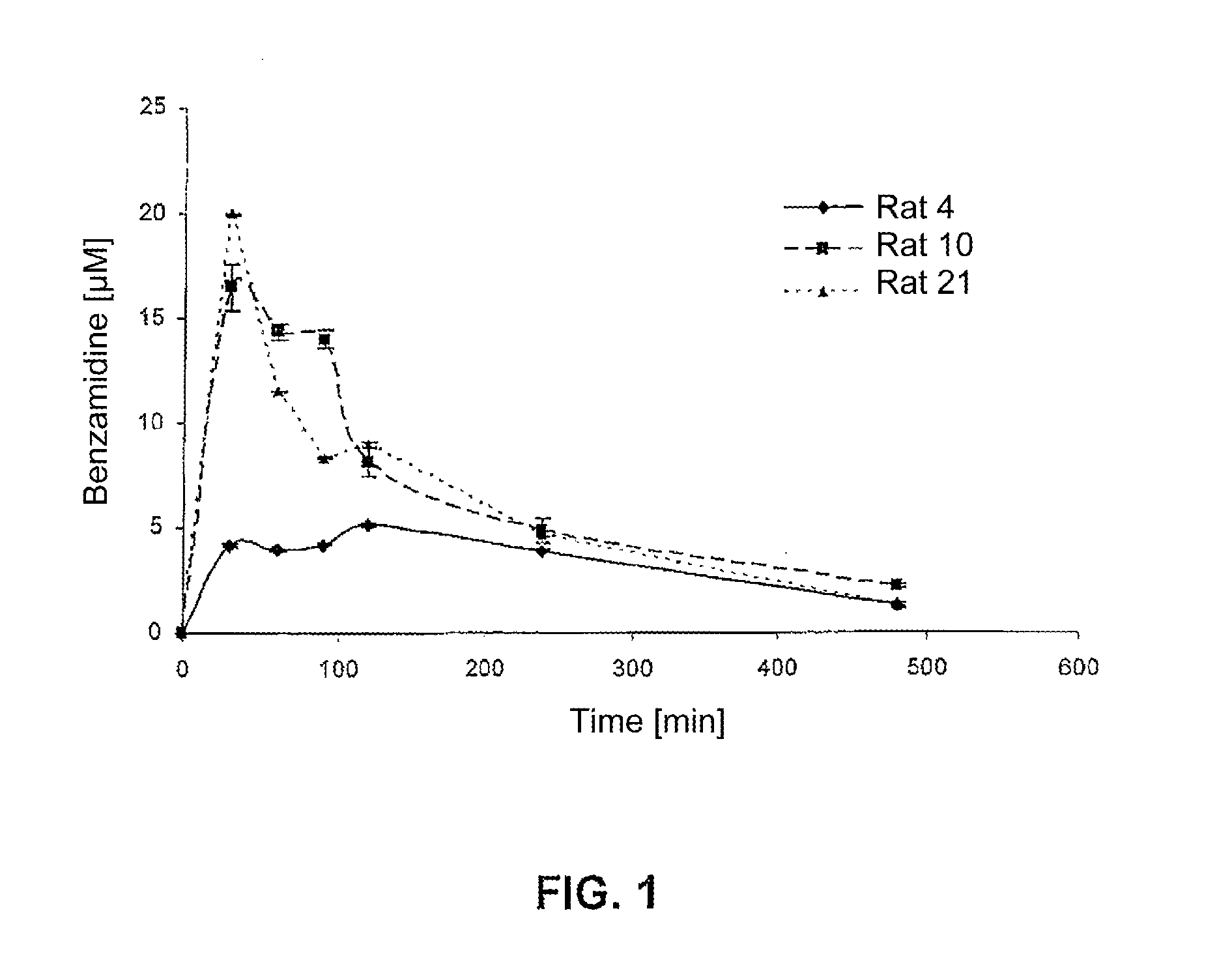
Examples
Embodiment Construction
[0031]Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood to one of ordinary skill in the art to which this invention pertains. Otherwise, certain terms used herein have the meanings as set in the specification. All patents, published patent applications and publications cited herein are incorporated by reference as if set forth fully herein. It must be noted that as used herein and in the appended claims, the singular forms “a,”“an,” and “the” include plural reference unless the context clearly dictates otherwise.
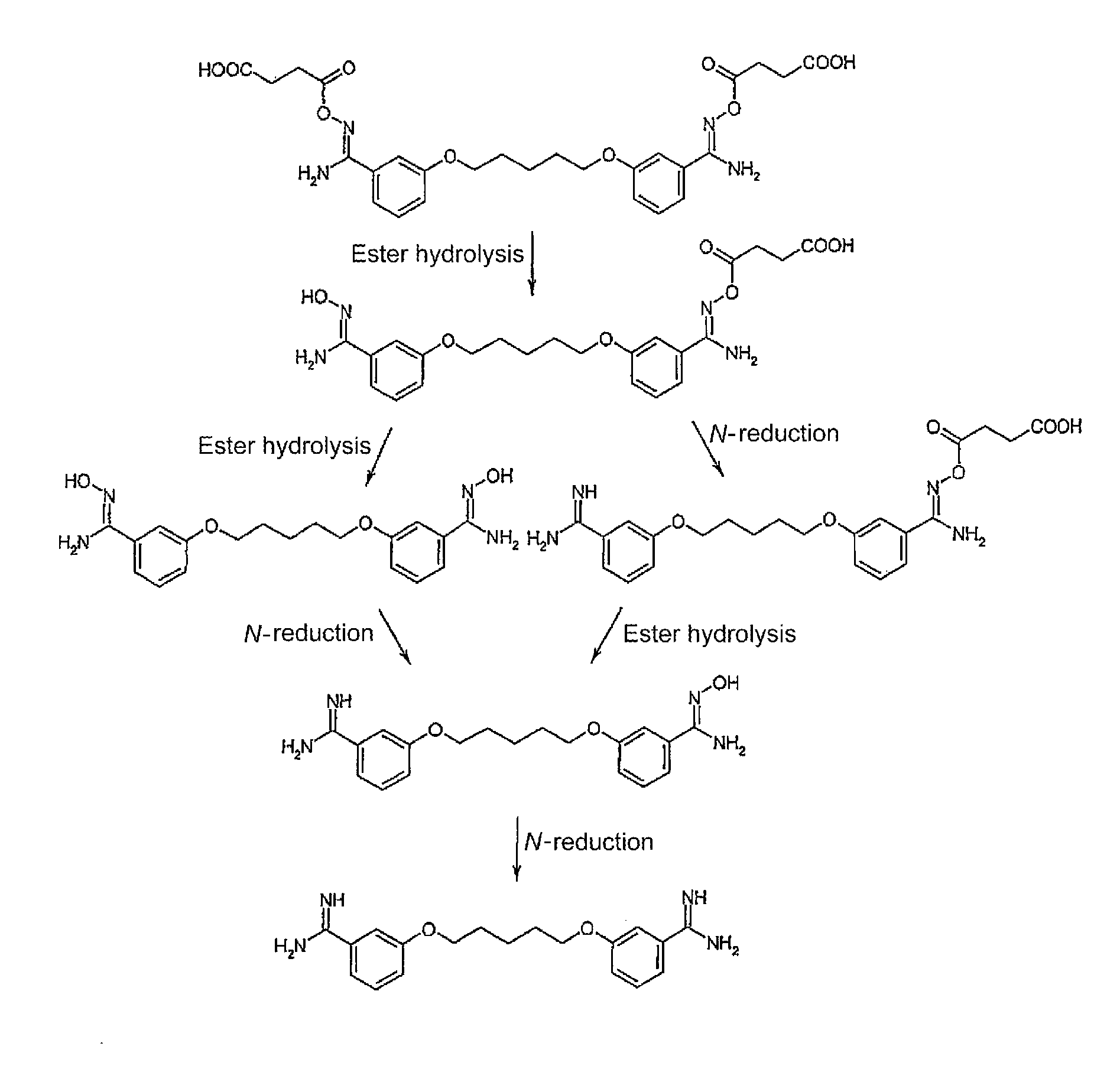
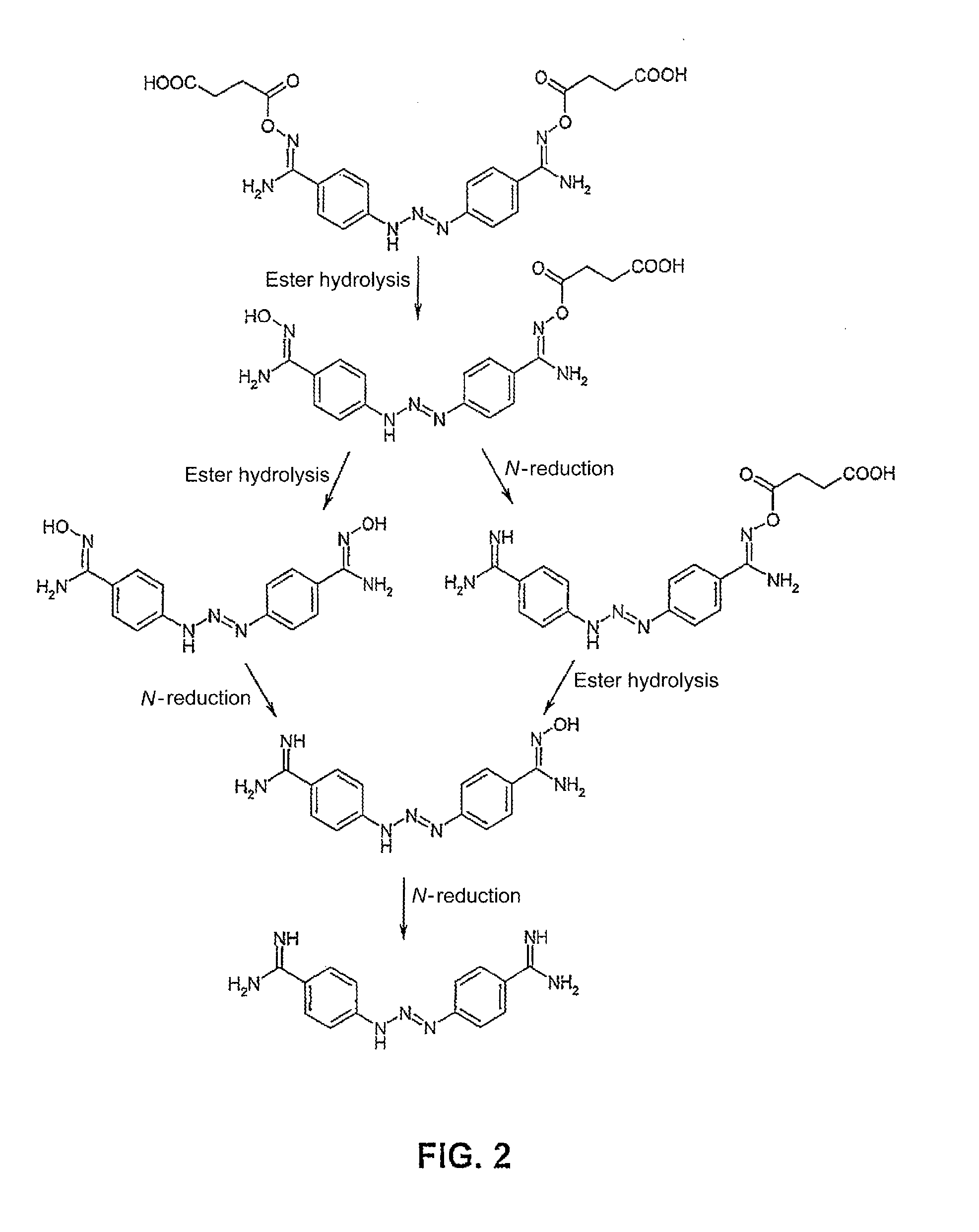
[0032]According to embodiments of the invention, amidoxime carboxylic acid esters (I) and the N-hydroxyguanidine carboxylic acid esters (II) of the following formulas are proposed to be used:
wherein n=0-12, and R1 represents hydrogen, an alkyl or aryl radical, or the salts thereof as a substitute for one or more amidine functions, N-hydroxyamidine (amidoxime) functions, guanidine functions or N-hydroxyguanid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com