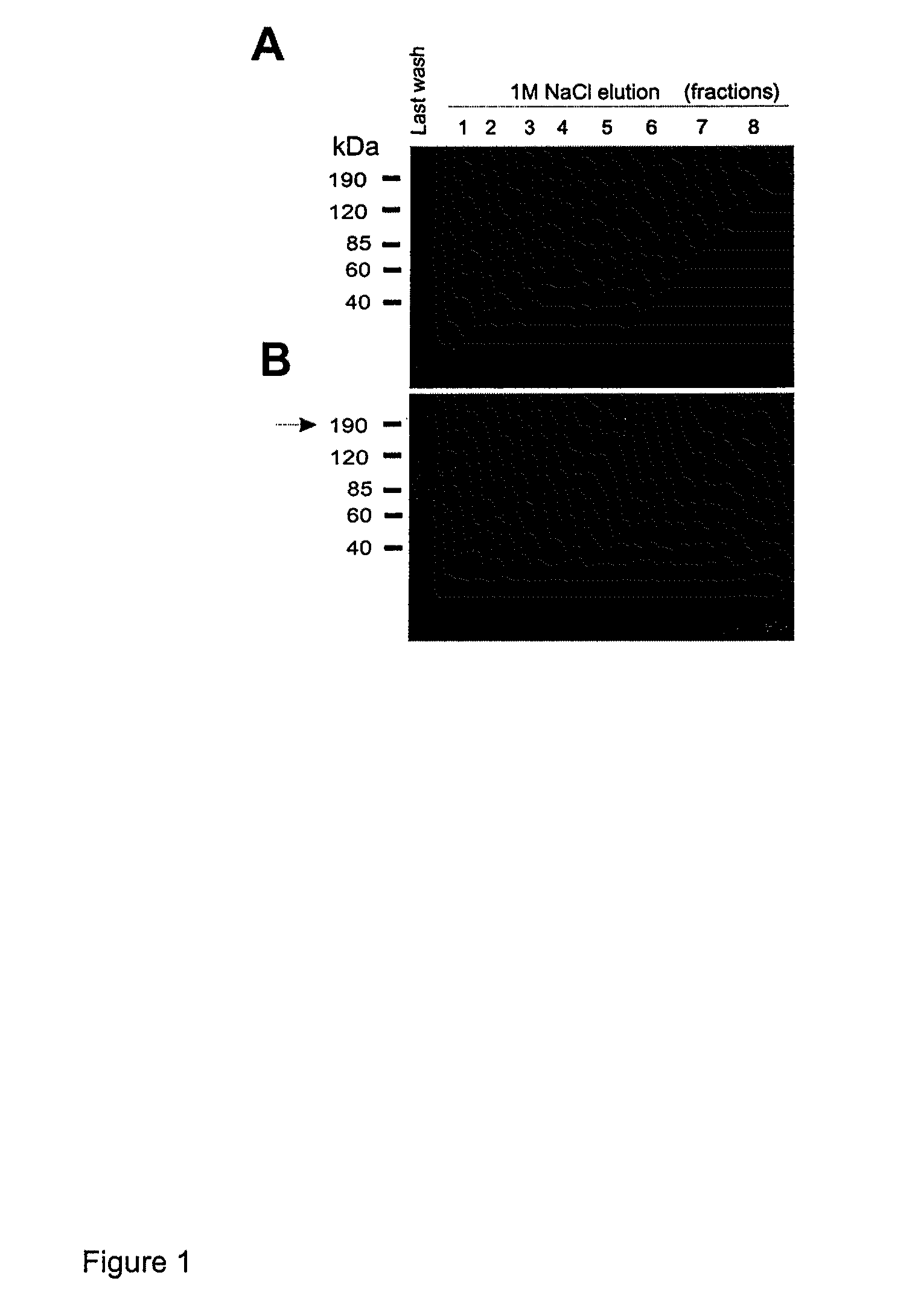
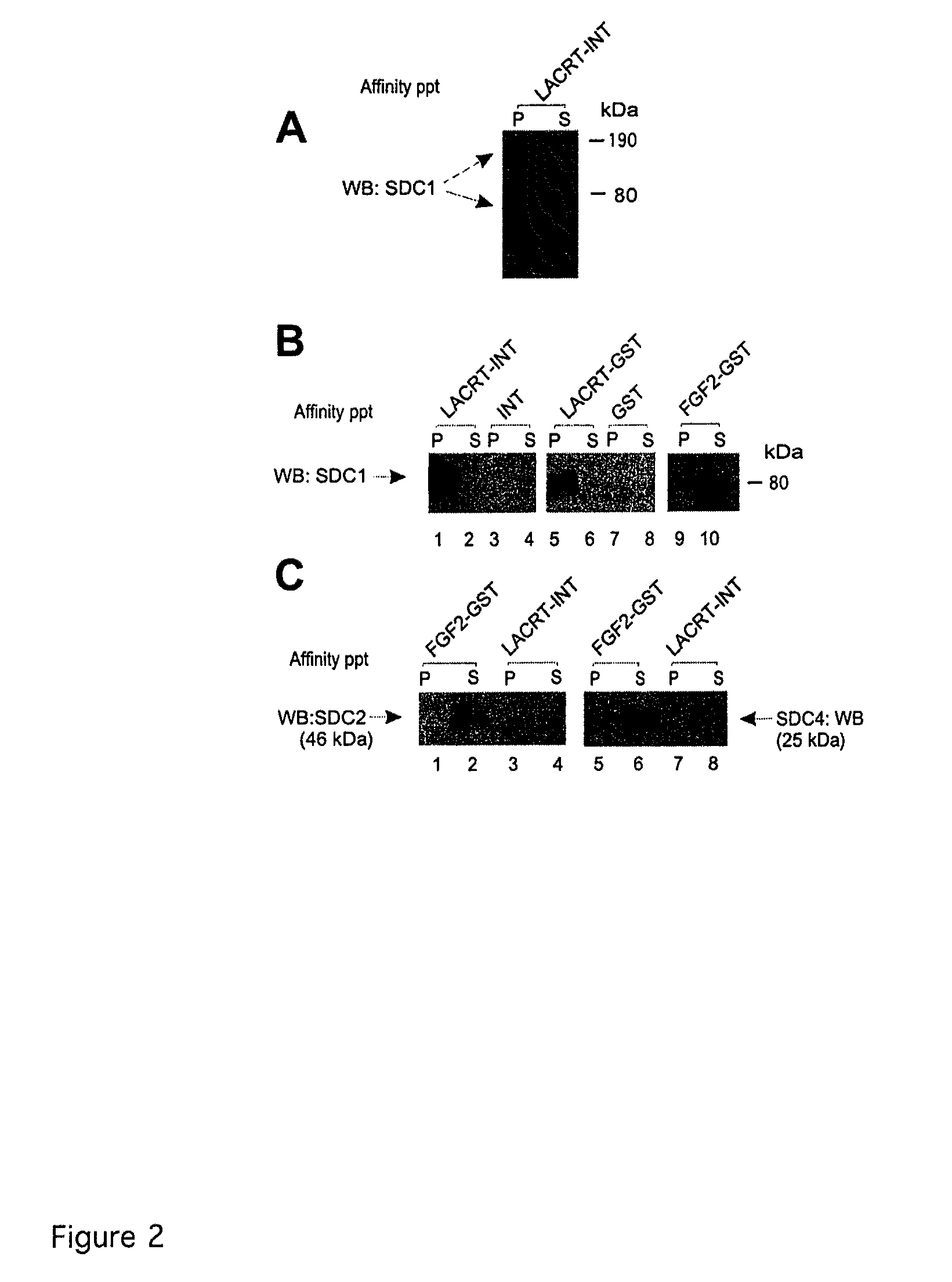
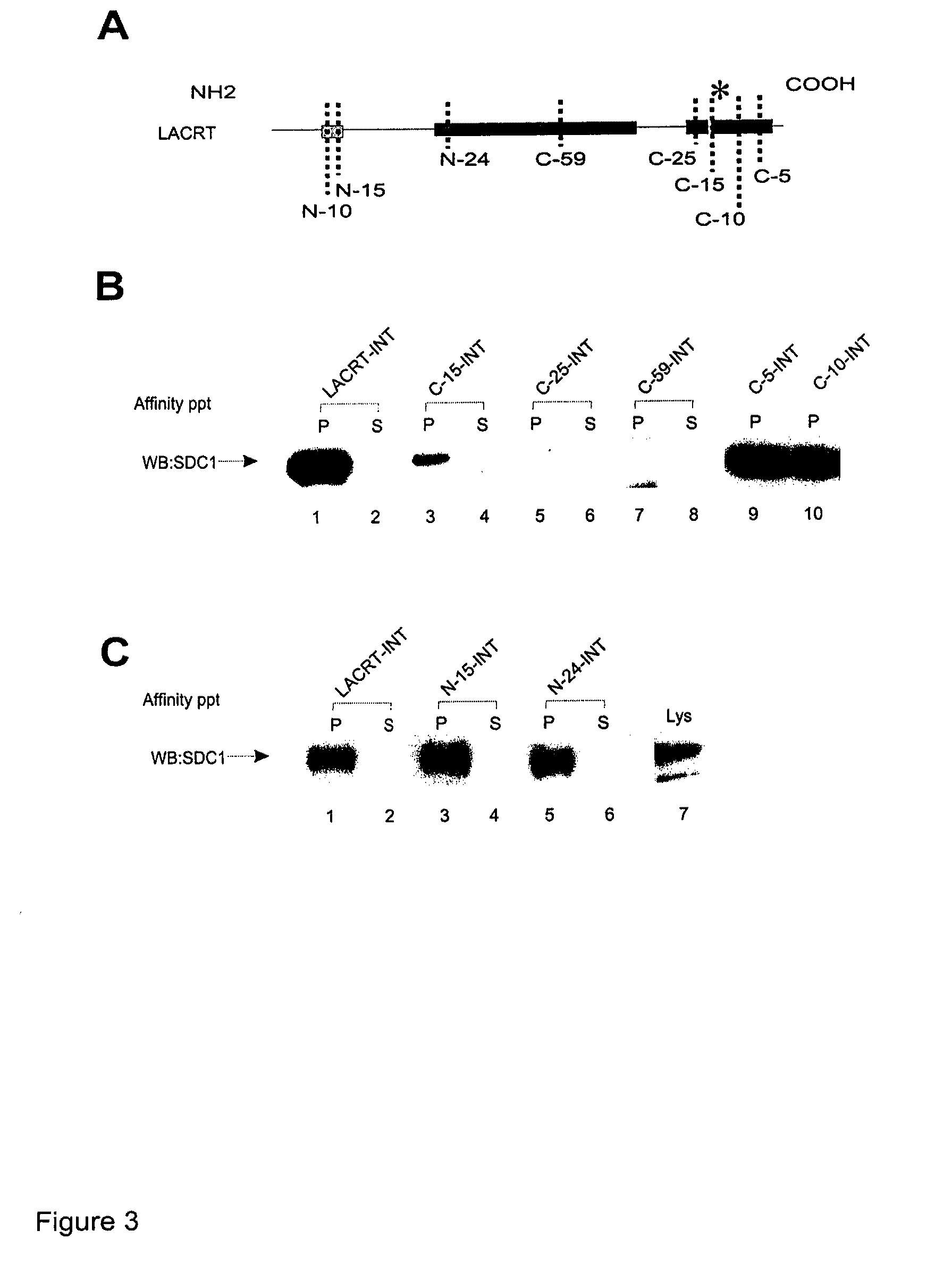
Lacritin-Syndecan Interactions
a technology of lacritin and syndecan, applied in the field of lacritinsyndecan interactions, can solve problems such as disrupting carcinoma activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Methods
[0173]Cell Culture, Plasmid Constructs and Transfection
[0174]The human salivary gland ductal (HSG) cell line was provided by Matthew Hoffman (NIDCR, Bethesda Md.). HSG cells were cultured in DMEM / F12 with 10% FBS. Cells were assayed between passage 10 and 20. Some HSG cells were transfected with a SMARTpool of four human SDC1 (Ambion Inc, Austin Tx) or heparanase-1 or heparanase-2 specific siRNAs at different doses (Dharmacon Inc, Lafayette Colo.). Other cells were transfected with individual siRNAs also at different doses. siRNAs sequences are as follows: (i) SDC1 siRNAs, CGACAAUAAACGGUACUUGTT, GGAGGAAUUCUAUGCCUGA, GGACUUCACCUUUGAAACCTT, and GGUAAGUUAAGUAAGUUGATT (gene bank accession no: NM—002997); SDC2 siRNAs, GGAGUUUUAUGCGUAAAACTT, GGAUGUAGAGAGUCCAGAGTT, and GGAGUGUAUCCUAUUGAUGTT (gene bank accession no: NM—002998); heparanase-1 siRNAs, GCAAUGAACCUAACAGUUUUU, GAUCAAACCUUGCCACCUUUU, GGACUGGACUUGAUCUUUGUU, and GAACAGCACCUACUCAAGAUU (gene bank accession no: NM—006665). Hepar...
example ii
[0209]Heparanase is an ‘on’ switch for lacritin binding to syndecan-1 (FIG. 12A; Ma et al, '06) that in turn appears to facilitate activation of a receptor. The receptor has the signaling characteristics of a Gαi or Gαo coupled receptor (GPCR; Wang et al, '06). For heparanase to play such a central role in lacritin cell targeting, one might expect heparanase to be a normal constituent of human tears. To the best of our knowledge heparanase has not been reported in tears. Collaborator Leslie Olsakovsky (UVa Ophthalmology) collected tears from normals and patients suffering from dry eye (mostly non-Sjögren's). Western blots of equal protein loads of 30 tear samples from normals vs dry eye patients suggest that heparanase is a normal tear constituent (see example blot FIG. 12B) and is substantially reduced in dry eye tears. Interestingly lacritin, UTP and ATP stimulate heparanase release (FIG. 12C). The 65 kDa form detected is the latent pro-survival form. Heparanase becomes active upo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Interaction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com