METHODS FOR PRODUCTION AND USE OF SUBSTANCE-LOADED ERYTHROCYTES (S-IEs) FOR OBSERVATION AND TREATMENT OF MICROVASCULAR HEMODYNAMICS
a technology of erythrocytes and erythrocytes, which is applied in the field of clinical use of erythrocytes, can solve the problems of rendering them incompetent—or at least inefficient—with regard, and achieve the effect of reducing hemoglobin and facilitating medical imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ad-Hoc Preparation of ICG Dye-Loaded Human S-IEs for Autologous Re-Injection
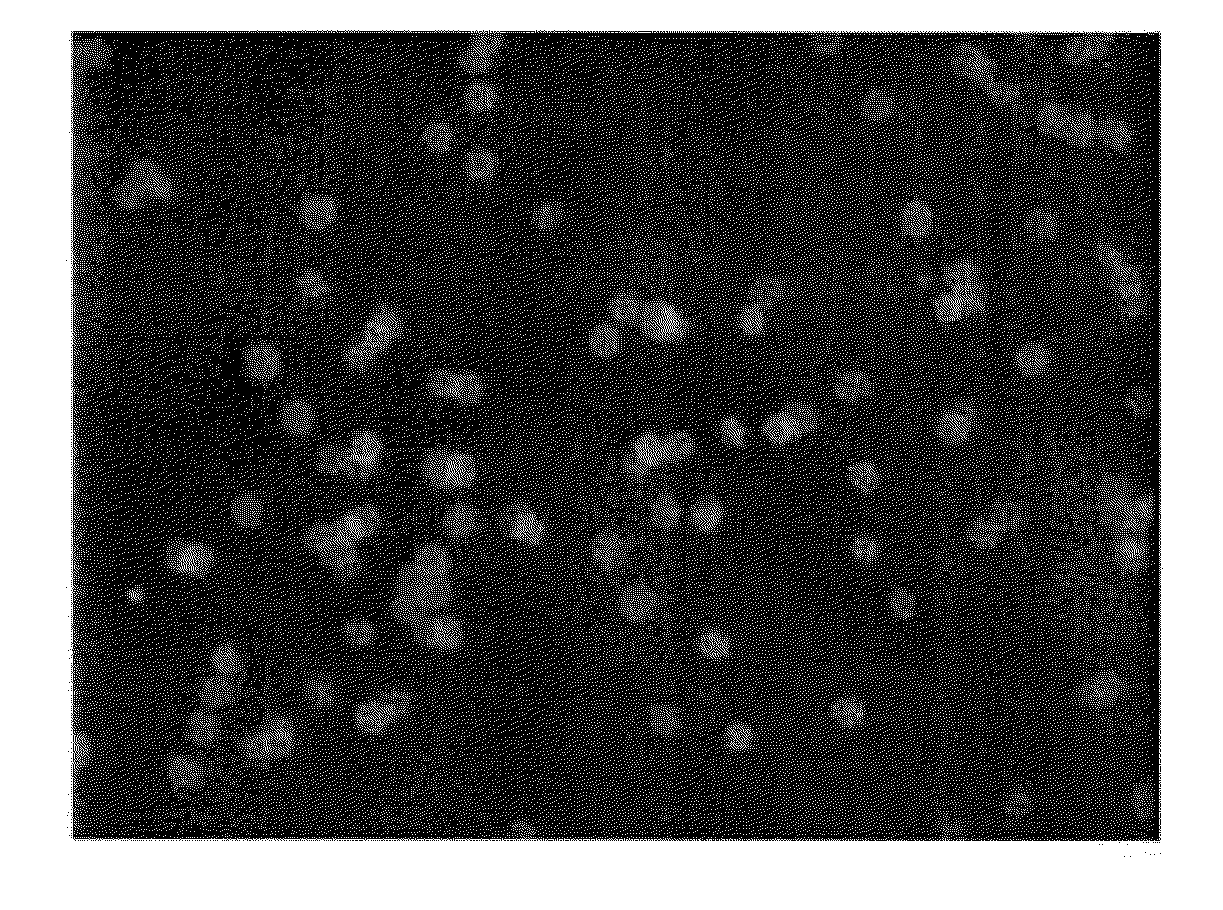
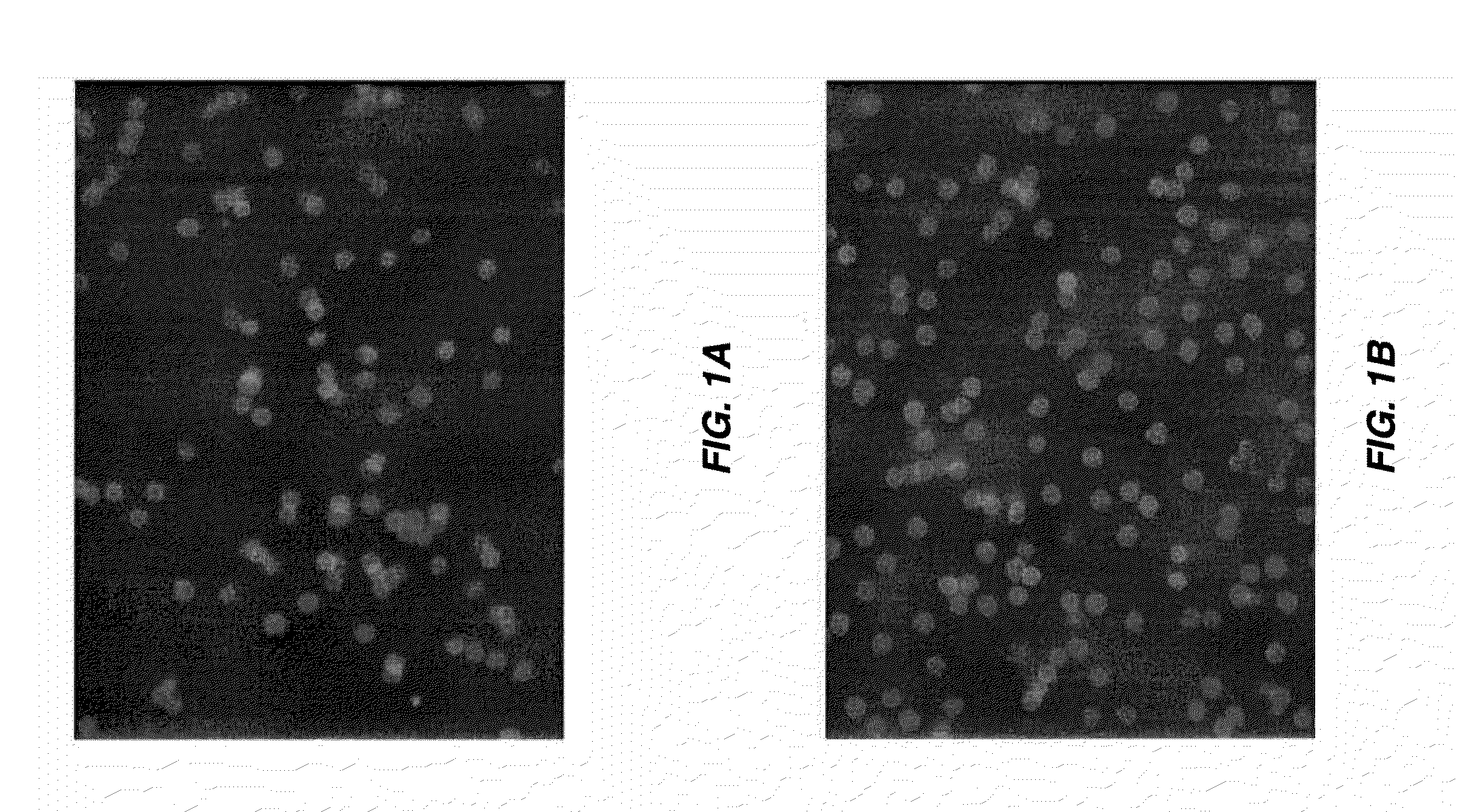
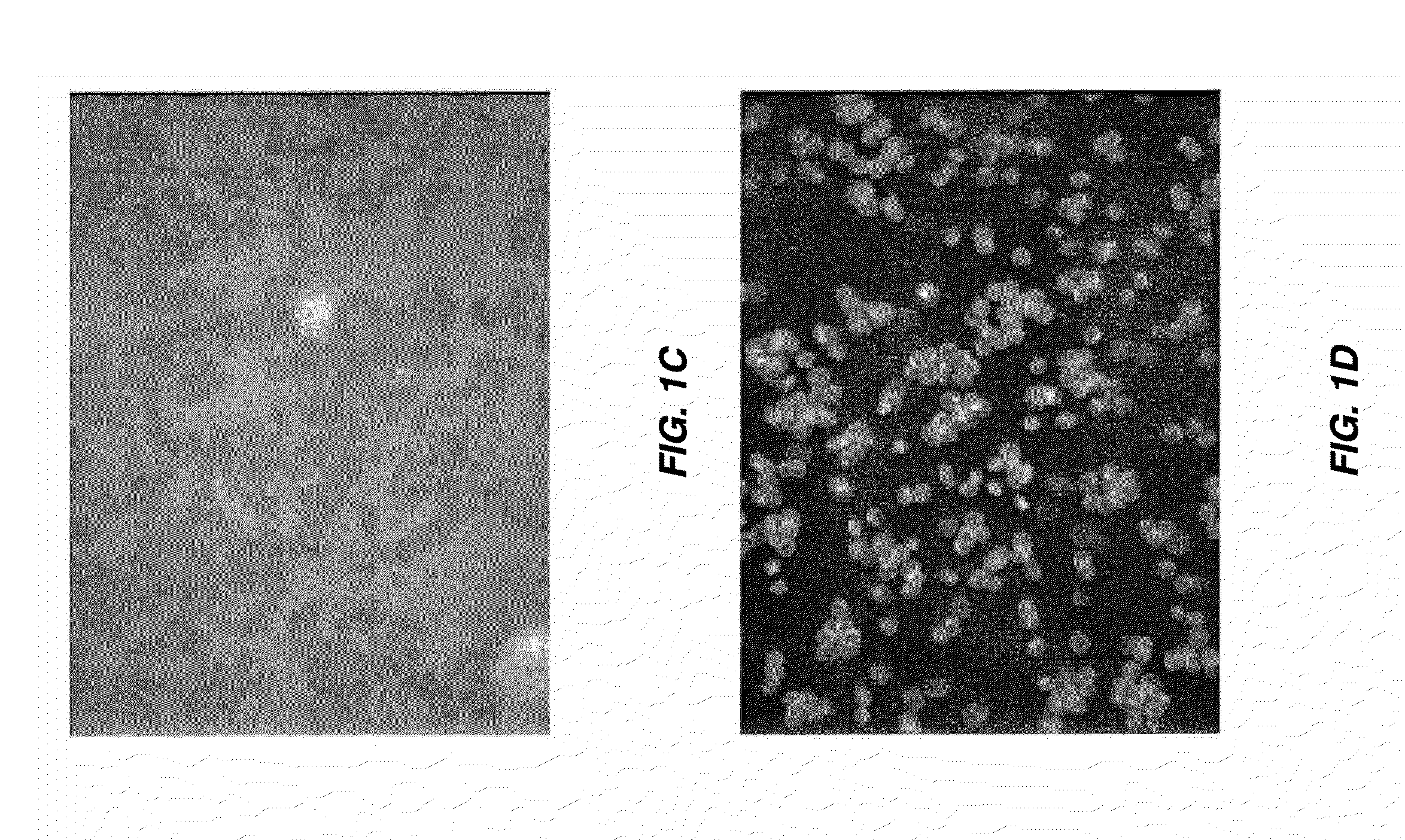
[0166]ICG is encapsulated in human erythrocytes by a procedure of hypotonic dialysis, isotonic resealing and re-annealing. These steps are carried out using the various sterile containers (pre-loaded with appropriate fluids) included in a kit, as depicted in FIG. 9. Some of the containers are centrifuge tubes, and all containers and vials are disposable. FIG. 9 is a diagram of the sterile containers used in substance loading of erythrocytes. A: one 10-mL vacutainer containing an anticoagulant for acquisition of a blood sample. B: represents two 15-mL and two 50-mL centrifuge vacutainer tubes, each containing a pre-measured amount of isotonic saline washing buffer. C: a container having a rubber stopper top to which a length of dialysis tubing (sealed at its bottom) is affixed, such that an erythrocyte-containing solution can be introduced into the dialysis tube via an injection needle inserted through the ru...
example 2
A Non-Dialysis Alternative to the Method in Example 1
[0173]ICG encapsulation in human erythrocytes by a procedure of hypotonic dialysis, isotonic resealing and re-annealing similar to the method described in Example 1 can be carried out without recourse to a dialysis step. This is done by decreasing the tonicity of the solution in which erythrocytes are suspended from 300 mOsm / kg to 87 mOsm / kg in four stages to open pores in the cells' membranes. These stages are all carried out in the same 50-mL centrifuge tube, at the completion of which ICG dye can be introduced to the cell suspension solution. As in Example 1, a kit consisting of various sterile containers and pre-measured fluids is utilised. In this case, the a kit has different components than indicated in the one depicted in FIG. 9; this kit consists of:[0174]1 10-mL vacutainer* containing an anti-coagulant (acid-citrate-dextrose)[0175]2 15-mL vacutainers, each containing 10 mL sterilised washing buffer[0176]1 50-mL vacutaine...
example 3
An Alternative to the Non-Dialysis Method in Example 2
[0194]Encapsulation in erythrocyte ghost cells of a fluorescent dye, along with other substances, can be carried out using a kit of pre-loaded, sterilized tubes consisting of:[0195]1 10-mL vacutainer* containing an anti-coagulant (acid-citrate-dextrose)[0196]1 15-mL vacutainer (empty)[0197]2 15-mL vacutainers each containing 11.5 mL of washing buffer[0198]1 15 mL vacutainer containing 2.0 mL of distilled H2O[0199]1 vial containing 1 mL of distilled H2O[0200]1 vial containing 29.8 mg of lyophilised ICG dye[0201]1 syringe pre-loaded with 1.5 mL sterilised distilled H2O for reconstitution of the ICG[0202]1 vial containing 260 μl of sterilised resealing solution[0203]1 vial containing 50 mL of sterilised washing solution[0204]3 long (about 4 inch) sterile needles
[0205]The method includes the steps of:[0206]Acquire 8.5 mL blood in B-D Vacutainer containing ACD-solution A (294 mOsm / kg, pH 7.4)[0207]The 8.5 mL sample is transferred to a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap