Molecular complexes which modify immune responses
a molecular complex and immune response technology, applied in the field of molecular complexes which modify immune responses, can solve the problems of complex generation of soluble divalent or multivalent molecular complexes comprising mhc class ii or t cell receptors (tcr), difficult to obtain them in proper folded form in absence of their respective integral membrane regions, and limited utility of these probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0100]This example demonstrates general construction and biochemical characterization of chimeric molecules.
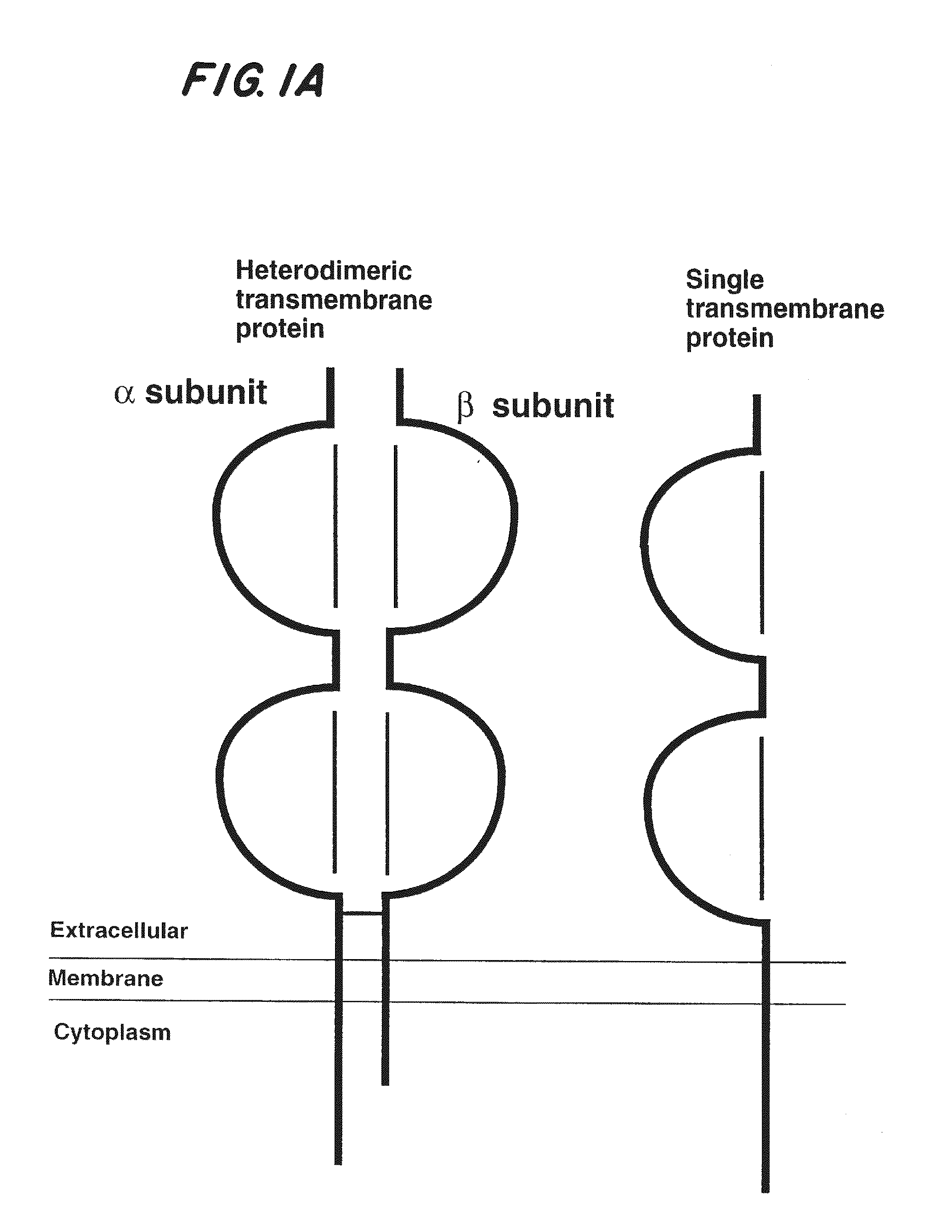
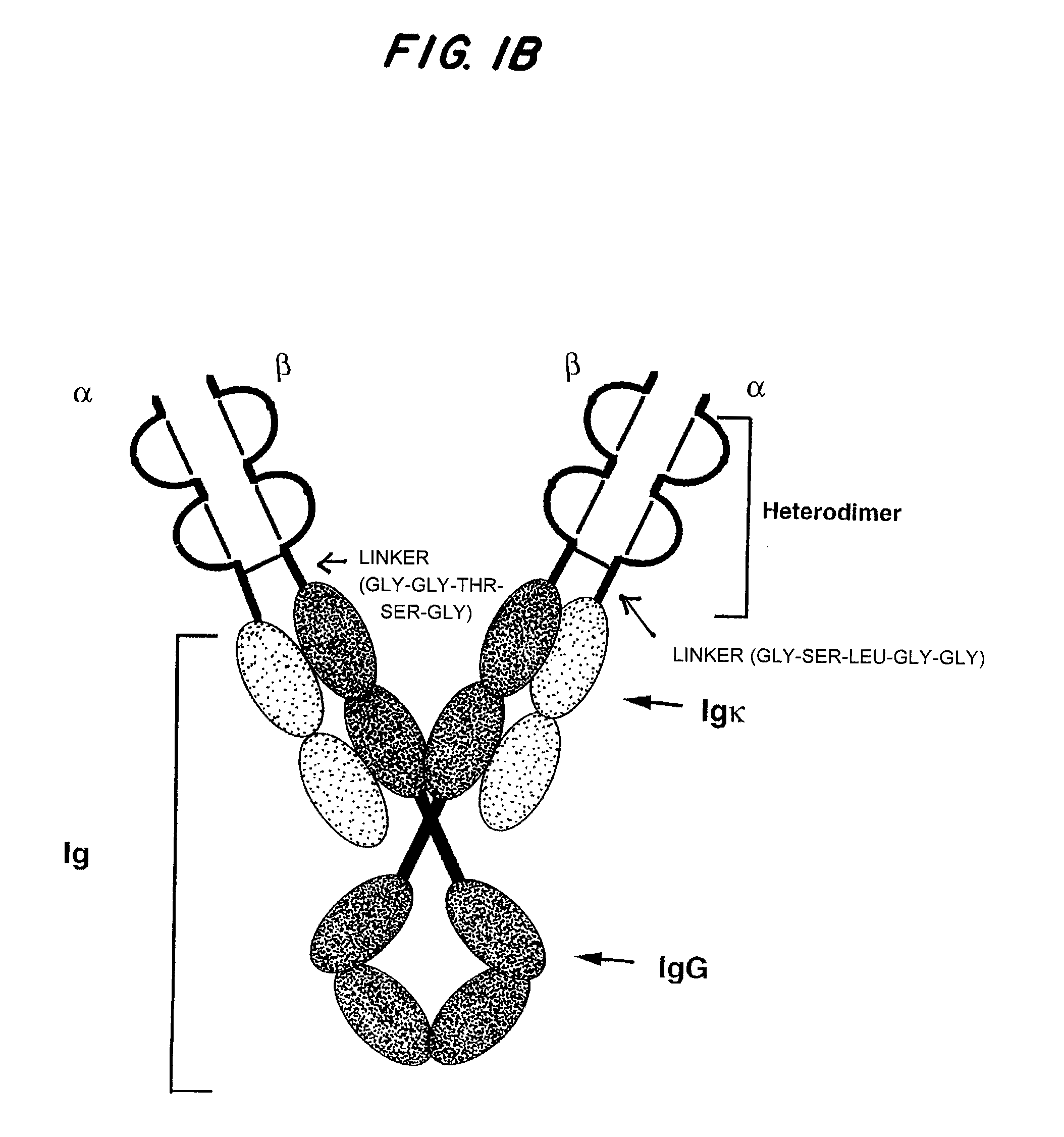
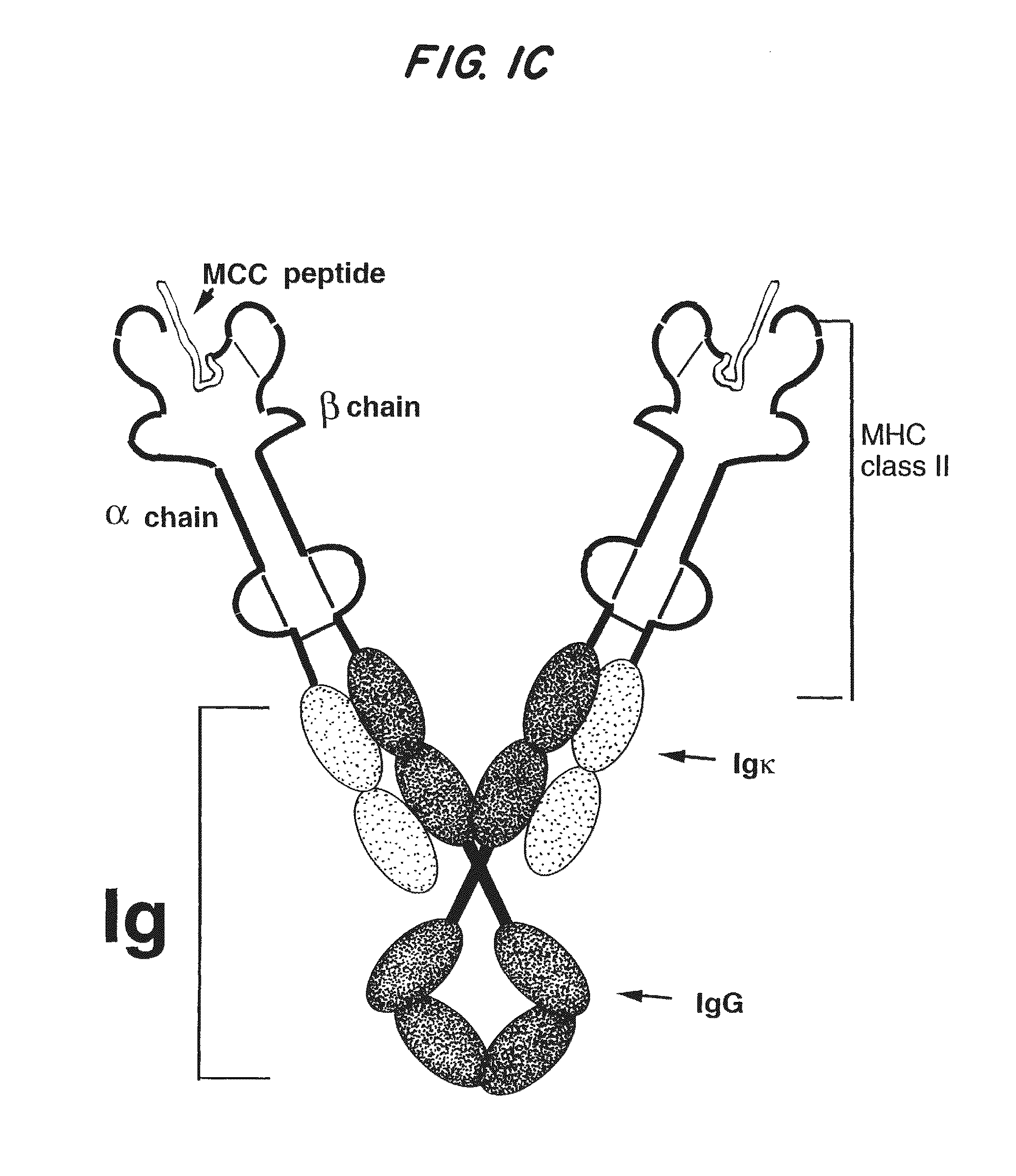
[0101]Characteristics of a general system for the expression of soluble divalent analogs of heterodimeric proteins include relative simplicity, broad applicability, and maintenance of molecular stability of the soluble analog. To accomplish this, IgG was chosen as a general molecular scaffold because it is divalent by nature and can be simply modified to serve as a scaffold (16, 26-28). Of further advantage is the fact that the IgG scaffold facilitates subunit pairing, folding, secretion, and stability of the covalently linked heterodimeric polypeptides.
[0102]Using immunoglobulin as a backbone, a general system has been designed for expression of soluble recombinant multivalent analogs of heterodimeric transmembrane proteins (FIGS. 1B-1D and FIG. 2). As shown in FIG. 2, site-directed mutagenesis was used to insert restriction enzyme sites, such as KpnI and Hind III, into the 5...
example 2
[0108]This example demonstrates detection of soluble heterodimeric proteins.
[0109]Cells infected with baculovirus containing transfer vectors encoding the soluble chimeric Ig constructs described above secrete a soluble chimeric Ig-like molecule detected by specific ELISA assays 4-5 days post infection. For 2C TCR / IgG, the assay was based on a primary antibody specific for murine IgG1 Fc (plated at 10 μg / ml) and a biotinylated secondary antibody, H57 (used at 1:5000 final dilution), specific for a conformational epitope expressed on the β chain of many TCR (FIG. 5A) or biotinylated 1B2 or a monoclonal antibody specific for a clontoypic epitope expressed on 2C TCR (FIG. 5B). For detection of I-E / IgG chimeric molecules, the same primary antibody was used and the biotinylated secondary antibody was 14.4.4, which is specific for I-E α chain (FIG. 5C). Supernatants from infected cells were incubated for 1 hour at room temperature. Plates were washed extensively with phosphate buffered sa...
example 3
[0112]This example demonstrates affinity measurements of soluble divalent TCR interaction with peptide / MHC complexes.
[0113]A competitive inhibition assay was developed to measure the affinity of soluble 2C TCR / Ig for peptide / MHC complexes. This assay, similar to one previously used to determine the affinity of soluble monovalent 2C TCR for peptide / MHC complexes (Schlueter et al., Journal of Molecular Biology 256:859-869 (1996), is based on mAb 30.5.7 binding to a region of the ×2 helix of H-2 Ld that overlaps with TCR receptor binding (Solheim et al., Journal of Immunology 154:1188-1197 (1995); Solheim et al., Journal of Immunology 150:800-811 (1993).
[0114]Briefly, affinities of 30.5.7 Fab fragments for RMA-S Ld cells were determined by direct saturation analysis of 30.5.7 Fab binding to cells analyzed by flow cytometry. Cells were incubated with increasing amounts of FITC labeled 30.5.7 Fab, and dissociation constants were estimated from a plot of 1 / MCF vs. 1 / [30.5.7 Fab]. Affiniti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com