Method and apparatus for the delivery of plynucleotide vaccines to mammalia skin
a technology of polynucleotide and mammalian skin, applied in immunological disorders, antibody medical ingredients, therapy, etc., can solve the problems of pulseagile electrical waveform, unobserved results, and each slow pulseagile electrical waveform administration protocol took approximately 3.5 seconds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
Purpose and Scope
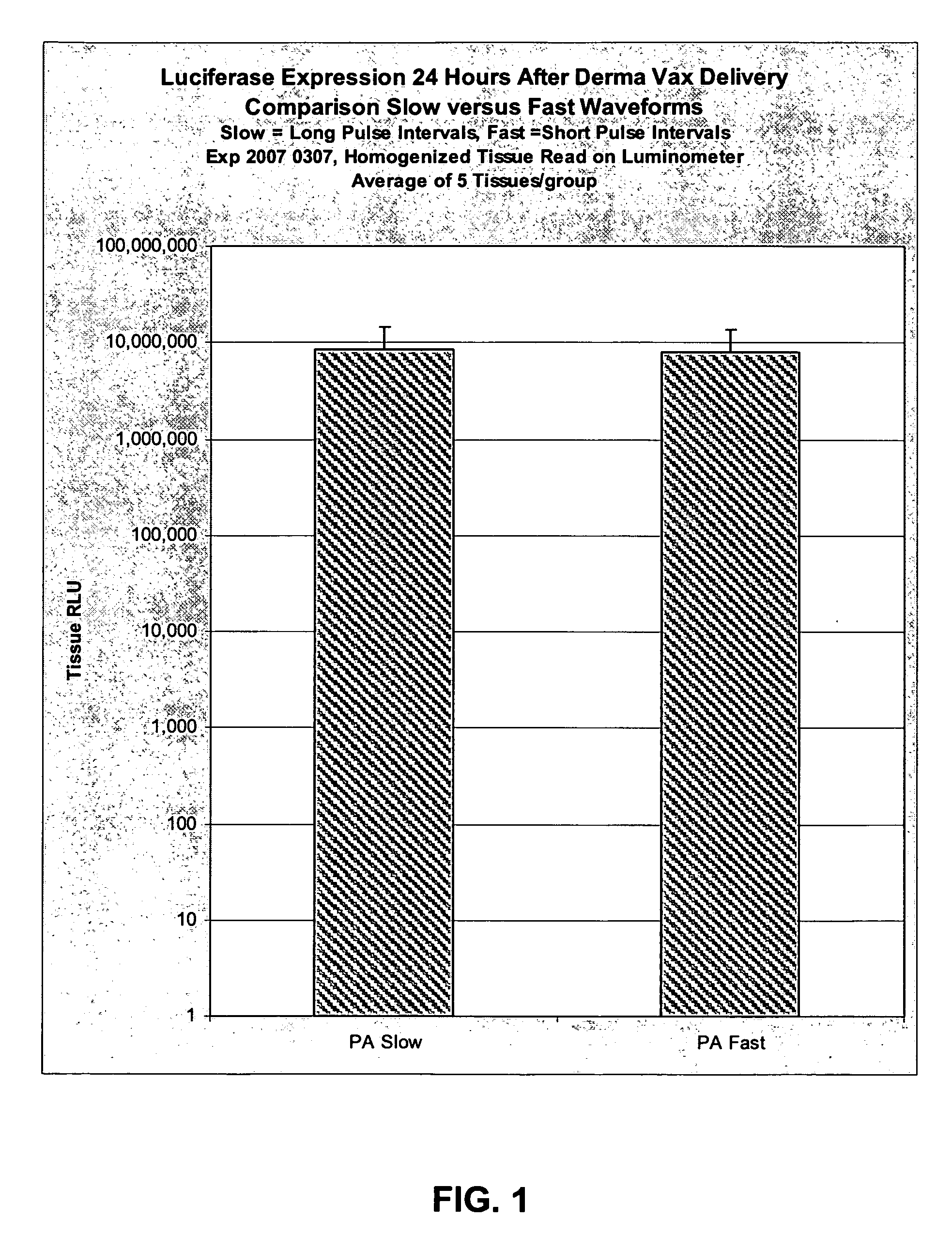
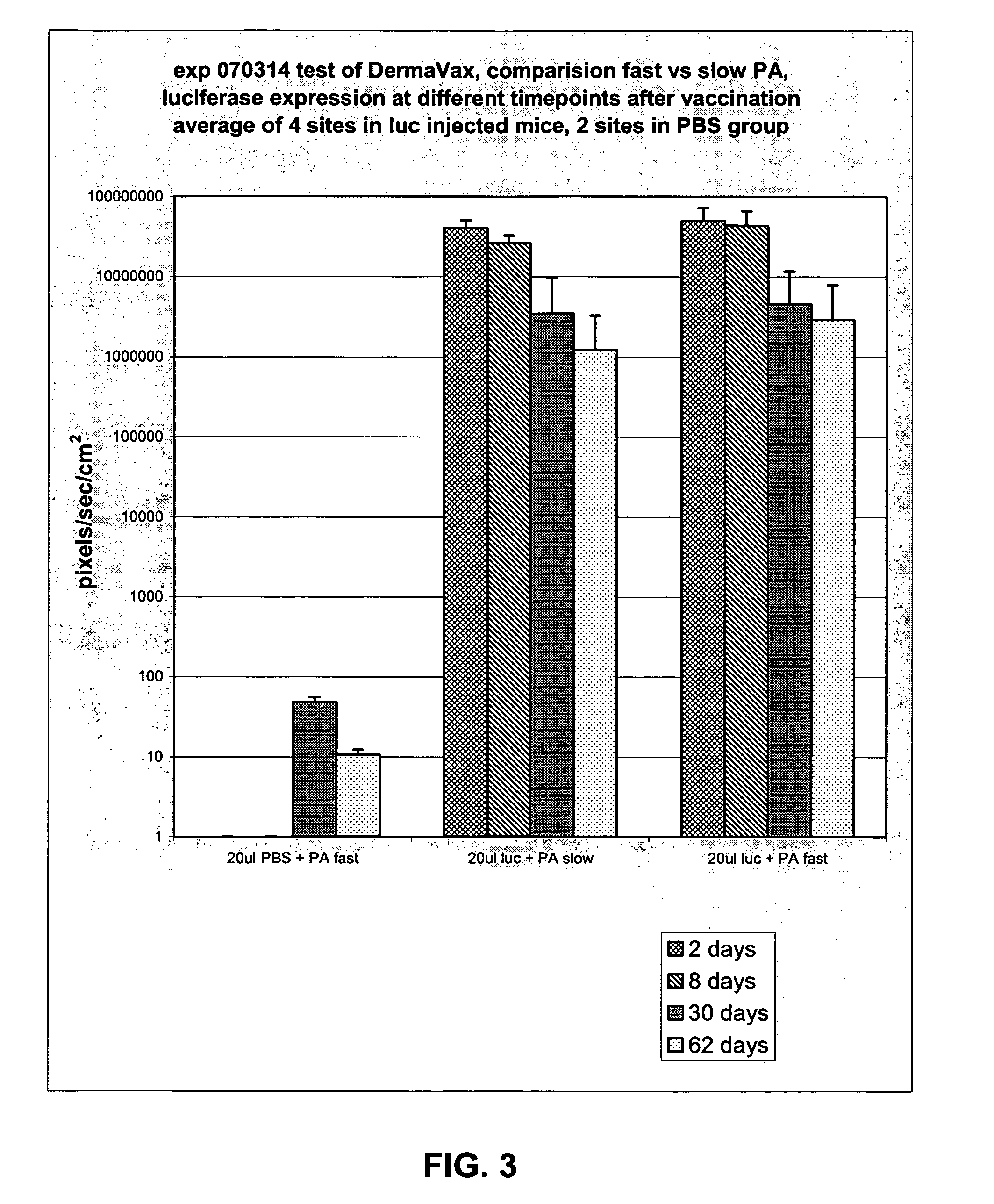
[0064]The purpose of this experiment is to compare fast PulseAgile electrical waveforms (using the Cyto Pulse “Derma Vax” system) versus slow PulseAgile electrical waveforms (using the Cyto Pulse PA-4000 system). The new Derma Vax system can deliver pulses more rapidly than the PA-4000.
Background
[0065]Dr. Anna-Karin Roos published at least two waveforms that induced good luciferase expression in the skin of mice. The system used was the PA-4000, and slow PulseAgile electrical waveforms were employed. New capabilities have been engineered into the Derma Vax system which employs the “CCEP-40 Waveform Generator”. One significant difference is that the Derma Vax system can deliver pulses with shorter pulse intervals. That is, with the “Derma Vax” system, pulse intervals of less than 100 milliseconds can be provided. This experiment will evaluate the effect on in vivo luciferase expression using fast PulseAgile electrical waveforms.
Approach
[0066]Plasmid used: gWizLucifer...
experiment 2
Purpose and Scope
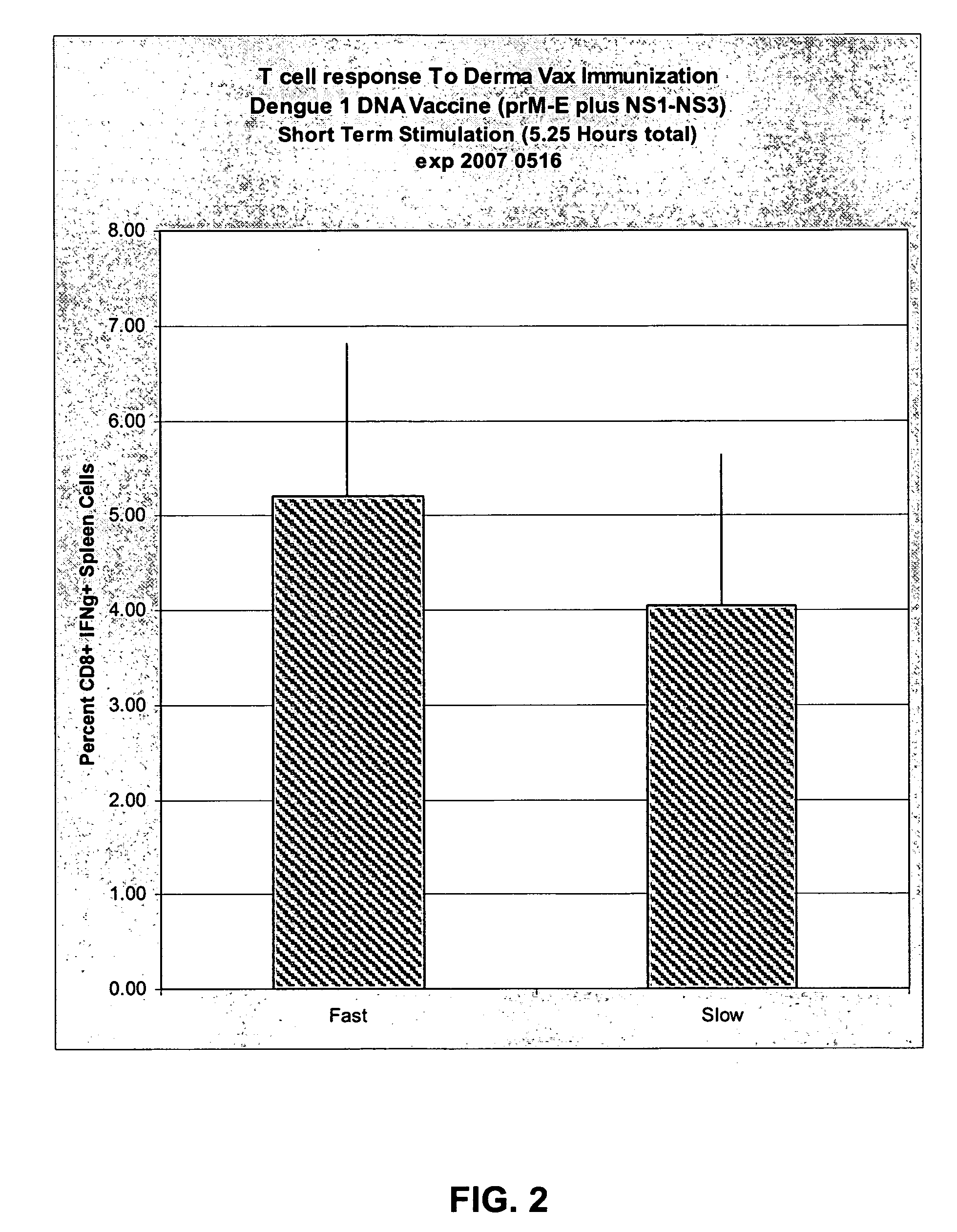
[0075]The purpose of this experiment is to compare T cell responses induced by DNA immunization using fast PulseAgile electrical waveforms (using the Cyto Pulse “Derma Vax” system) versus slow PulseAgile electrical waveforms (using the Cyto Pulse PA-4000 system). The new Derma Vax system can deliver pulses more rapidly than the PA-4000. More specifically, the purpose of this study is to compare T cell responses induced by DNA immunization using Pulse Agile Derma Vax delivery with Dengue 1 plasmids expressing prM-E and NS1-NS3.
Background
[0076]Dr. Anna-Karin Roos published at least two waveforms that induced good luciferase expression in the skin of mice. The system used was the PA-4000, and slow PulseAgile electrical waveforms were employed. New capabilities have been engineered into the Derma Vax system which employs the “CCEP-40 Waveform Generator”. One significant difference is that the Derma Vax system can deliver pulses with shorter pulse intervals. That is, wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| waveform width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com