Method for the generation of monoclonal antibodies derived from human b cells
a monoclonal antibody and human b cell technology, applied in the field of human b cells, can solve the problems of low cloning efficiency, elusive efficient method of obtaining such antibodies, and low efficiency of immortalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Experimental Details
Preparation of Complete Medium
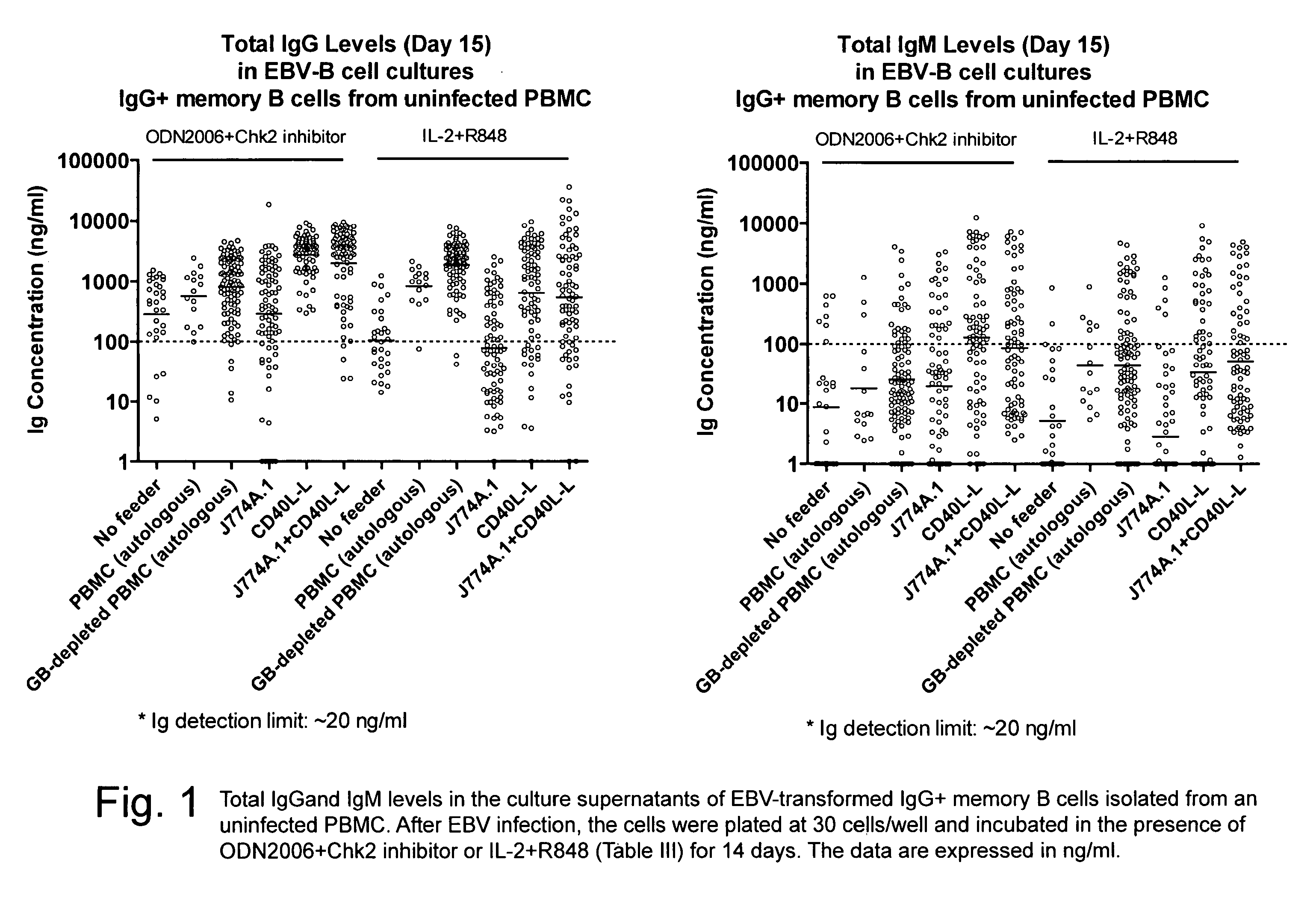
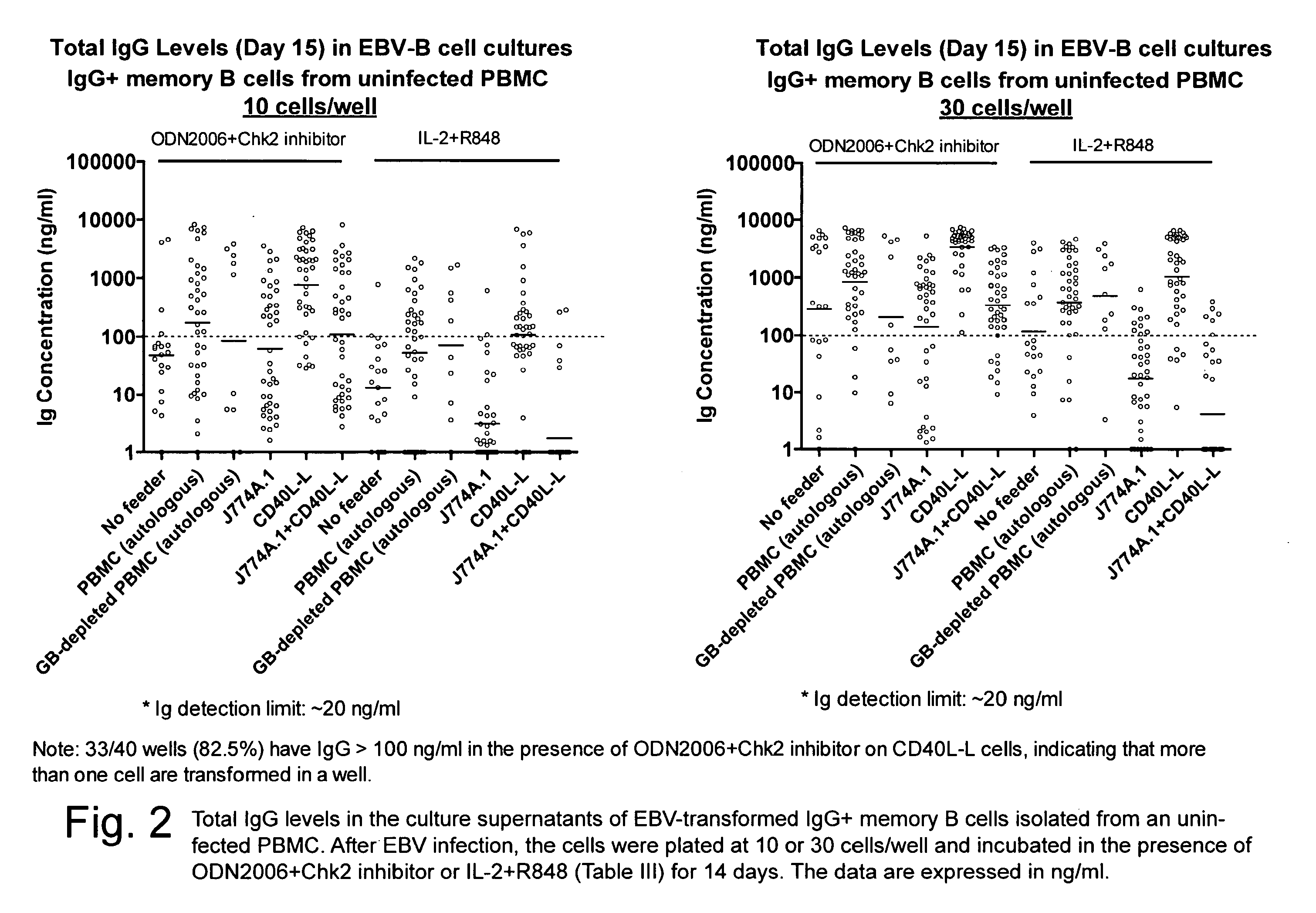
[0029]Complete Medium (CM) for PBMC, EBV-B cells, J774A.1, and K6H6 / B5 cell lines
[0030]RPMI 1640 (Invitrogen) supplemented with:[0031]1) 15.2% heat-inactivated fetal calf serum (FCS)[0032]2) 1% non-essential amino acids (NEAA)[0033]3) 1 mM sodium pyruvate[0034]4) 15 mM HEPES buffer, pH 7.3[0035]5) 2 mM L-glutamine (Glu)[0036]6) 100 U / ml penicillin G (Pen)[0037]7) 100 μg / ml streptomycin (Strep)
[0038]Preparation:[0039]1. Thaw a bottle of FCS (500 ml / bottle, −20° C.) and incubate at 56° C. for 30 min for heat inactivation (inactivation of c′)[0040]2. Let bottle cool down and add 31 ml of Glu+Pen / Strep (100×, 100 ml / bottle, −20° C.)[0041]3. Aliquot the FCS-antibiotics mixture into tubes at 50 ml / tube and freeze at −20° C.[0042]4. Add 100 ml of FCS-antibiotics mixture to bottle of RPMI 1640 (500 ml / bottle, 4° C.)[0043]5. Add 6.2 ml of HEPES buffer (100×, 100 ml / bottle, 4° C.) into the bottle[0044]6. Add 6.2 ml of sodium pyruvate (100×, 1...
experiment 8-28
[0136]
FrequencySampleof positiveCDR3IDPCR wellsVDHJMutatedlengthProductive113 / 20 1~69*023~3*014*020.0372499F211 / 20 4~39*066~13*011*010.04301015F1 / 203~49*032~2*01 / inv, 02 / inv4*020.0925372N34 / 203~23*012~2*01 / inv, 02 / inv3*020.02949117F43 / 204~39*012~21*025*010.05221919F53 / 202~5*104~17*014*020.04143613F613 / 20 4~31*030~IR*01C4*020.02193810F73 / 204~34*012~2*01, 024*020.00266718F88 / 204~59*012~2*024*010.03753418FTubeOriginalSortCDR3IgIDWellWellVDHJMutatedlengthIsotypeProductive1B7A101~69*023~3*014*020.0374639779G1F1B7A101~69*023~3*014*020.0372492849G1F1B7A101~69*023~3*014*020.0373563229G1F1B7A21~69*023~3*014*020.0401146139G1F1B7A21~69*023~3*014*020.0401146139G1F1B7A21~69*023~3*014*020.0402298859G1F1B7A31~69*022~2*01, 021*010.0744985679MN1B7A31~69*023~3*014*020.0372492849G1F1B7A31~69*023~10*01 / inv1*010.0442477889A1N1B7A41~69*023~3*014*020.0372492849G1F1B7A41~69*023~3*014*020.0372492849G1F1B7A41~69*023~3*014*020.0373563229G1F1B7A51~69*023~3*014*020.0429799439G1F1B7A51~69*023~3*014*020.037249284...
experiment 8-29
Experiment 8-30—Total IgG ELISA
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| cell densities | aaaaa | aaaaa |
| fluorescent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com