Anti-galectin-1 (GAL1) monoclonal antibodies and fragments thereof for neutralizing gal1
a monoclonal antibody and anti-gal1 technology, applied in the field of anti-gal1 (gal1) monoclonal antibodies and fragments thereof, can solve the problems of poor event-free survival and little evidence of an effective host anti-tumor immune response, and achieve the effect of preventing or delaying the ons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Neutralizing Anti-Gal1 Monoclonal Antibodies Useful for Therapeutic Applications
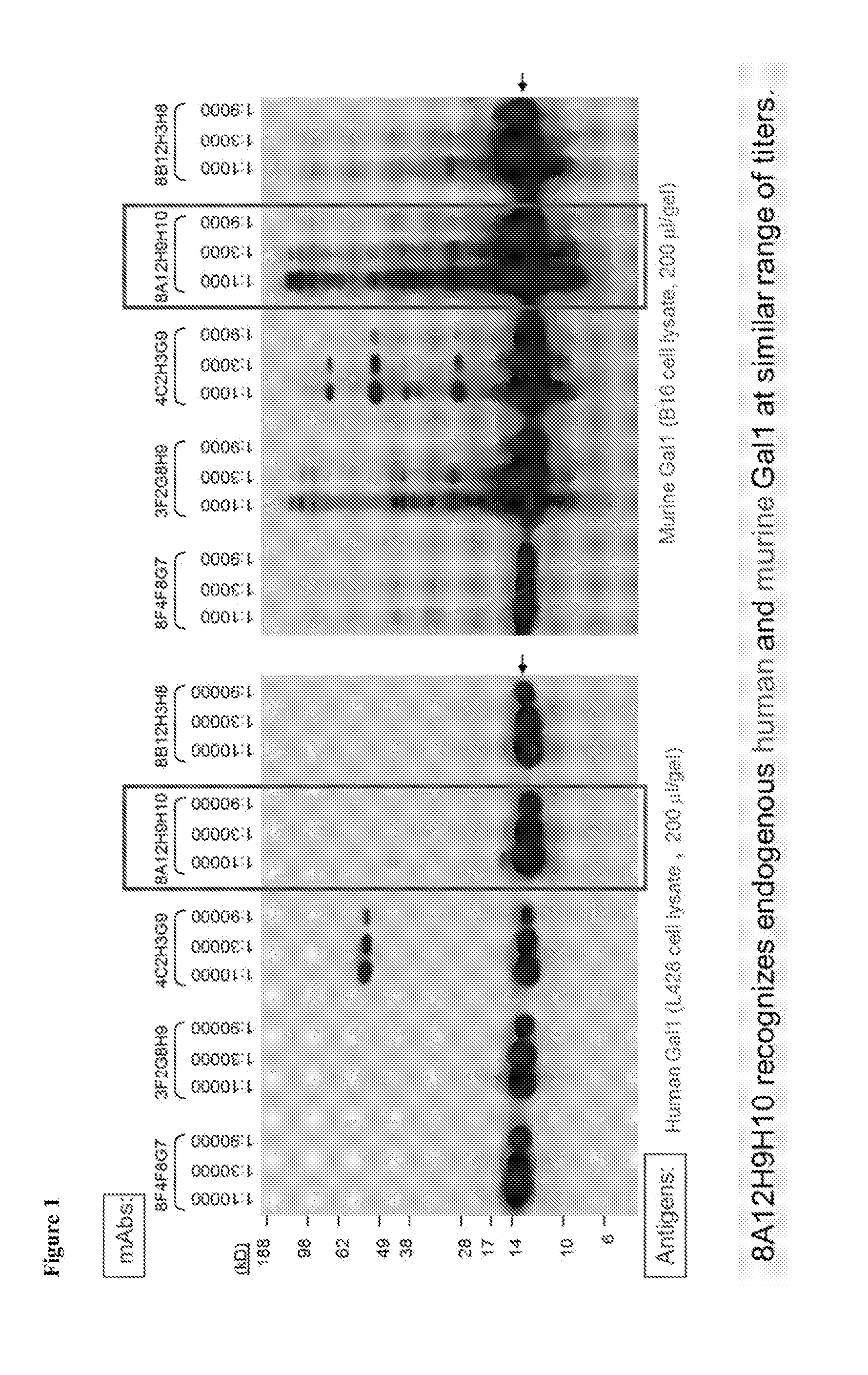
[0259]Neutralizing anti-Gal1 monoclonal antibodies were generated and reacted with human recombinant Gal1 and endogenous Gal1 in biochemical assays and in immunohistochemical analyses of primary tumors. In addition, several of the Gal1 monoclonal antibodies also cross-reacted well with endogenous Gal1 from cynomologous monkey and mouse (FIG. 1). Epitope mapping indicated that the 8B5, 8F4 and 8G3 Gal1 monoclonal antibodies all recognized a domain distal to the previously described carbohydrate-binding domain (FIG. 2 and Table 1).
[0260]These antibodies (i.e., 8B5, 8F4, and 8G3) were subsequently sequenced and determined to each have the same sequence, with the light chain being lambda. Briefly, total RNA was extracted from each hybridoma and subjected to RT-PCR using constant region specific 3′ primers and pools of degenerate signal sequence specific 5′ primers. Amplified products were cloned and sequence...
example 2
Fine Epitope Mapping of Neutralizing Anti-Gal1 Monoclonal Antibodies Useful for Therapeutic Applications
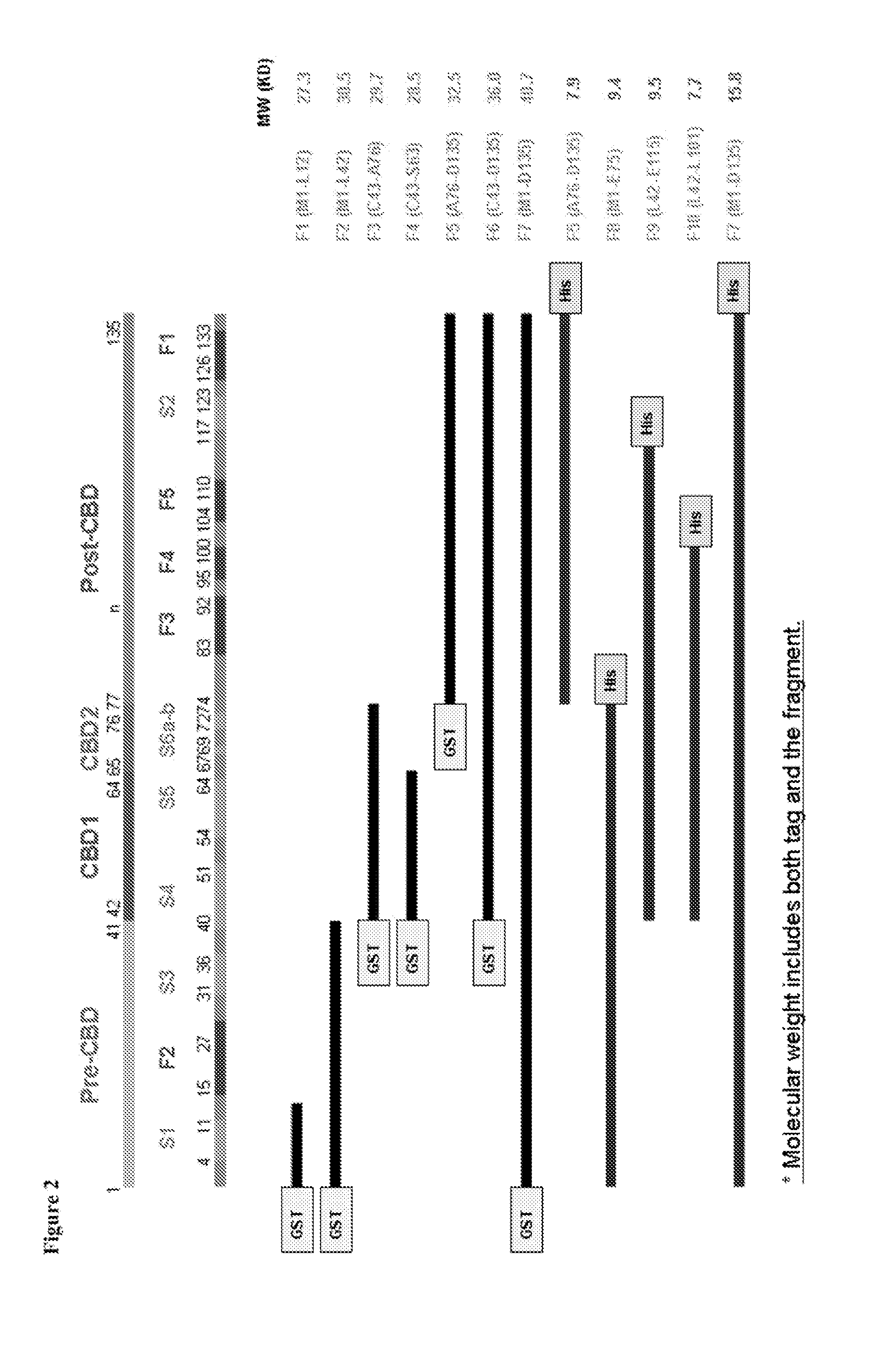
[0262]The 8F4 mAb was determined to cross-react well with both human Gal1 and mouse Gal1 in FIG. 1 and recognize a post-CBD domain of Gal1 in Table 2 were further subjected to fine epitope mapping analyses. In addition to the seven GST-tagged human Gal1 constructs shown in FIG. 2 and produced in E. coli, five additional 6×HIS-tagged human Gal1 constructs spanning various portions of the human Gal1 polypeptide were generated in E. coli for use in epitope mapping analyses (FIG. 2). FIG. 2 further demonstrates how the amino acids encompassed by each GST-tagged and HIS-tagged construct maps with respect to the β-strands in the five-stranded β-sheets (F1-F5) and six-stranded β-sheets (S1-S6a / S6b) of the folded human Gal-1 polypeptide (FIG. 3). The Gal1-neutralizing 8F4 mAb was determined to recognize recombinant HIS-F7, HIS-F5, and HIS-F9 by Western blot analysis, whereas an anti-Gal1,...
example 3
Biophysical Properties of Neutralizing Anti-Gal1 Monoclonal Antibodies Useful for Therapeutic Applications
[0263]Surface Plasmon Resonance (SPR) analyses (also called Biomolecular Interaction Analysis, BIAcore) were also conducted in order to further define the biophysical properties (e.g., kon, koff, koff / kon, (KD) of Gal1's interaction with the 8F4 mAb. SPR experiments were performed at 25° C. in the standard BIAcore running buffer HBS-EP on a BIAcore 3000) Instrument (BIAcore). In brief, anti-mouse antibody was first captured on the CM-5 sensor chip (GE HealthCare). Afterwards, approximately 250 response units (RU) of the 8F4 anti-Gal1 mAb were immobilized (with exception of ˜350 RU for rmGal1 assay) and followed by various dilutions of recombinant galectin (human galetin-1,2, 3, 4, 7, 8, 9 or murine galectin-1 (mGal1), from R&D Systems) to assess the binding of galectin to 8F4. All data are shown after subtraction from a channel loaded with buffer alone. Data analysis to obtain t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| immunogenic composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com