Phenoxy thiophene sulfonamides and other compounds for use as inhibitors of bacterial glucuronidase
a technology of phenoxy thiophene sulfonamide and other compounds, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, instruments, etc., can solve the problems of double-strand dna breakage and apoptosis, poor bioavailability and toxic side effects, and severe limitation of cpt-11 efficacy, so as to prevent dose-limiting diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
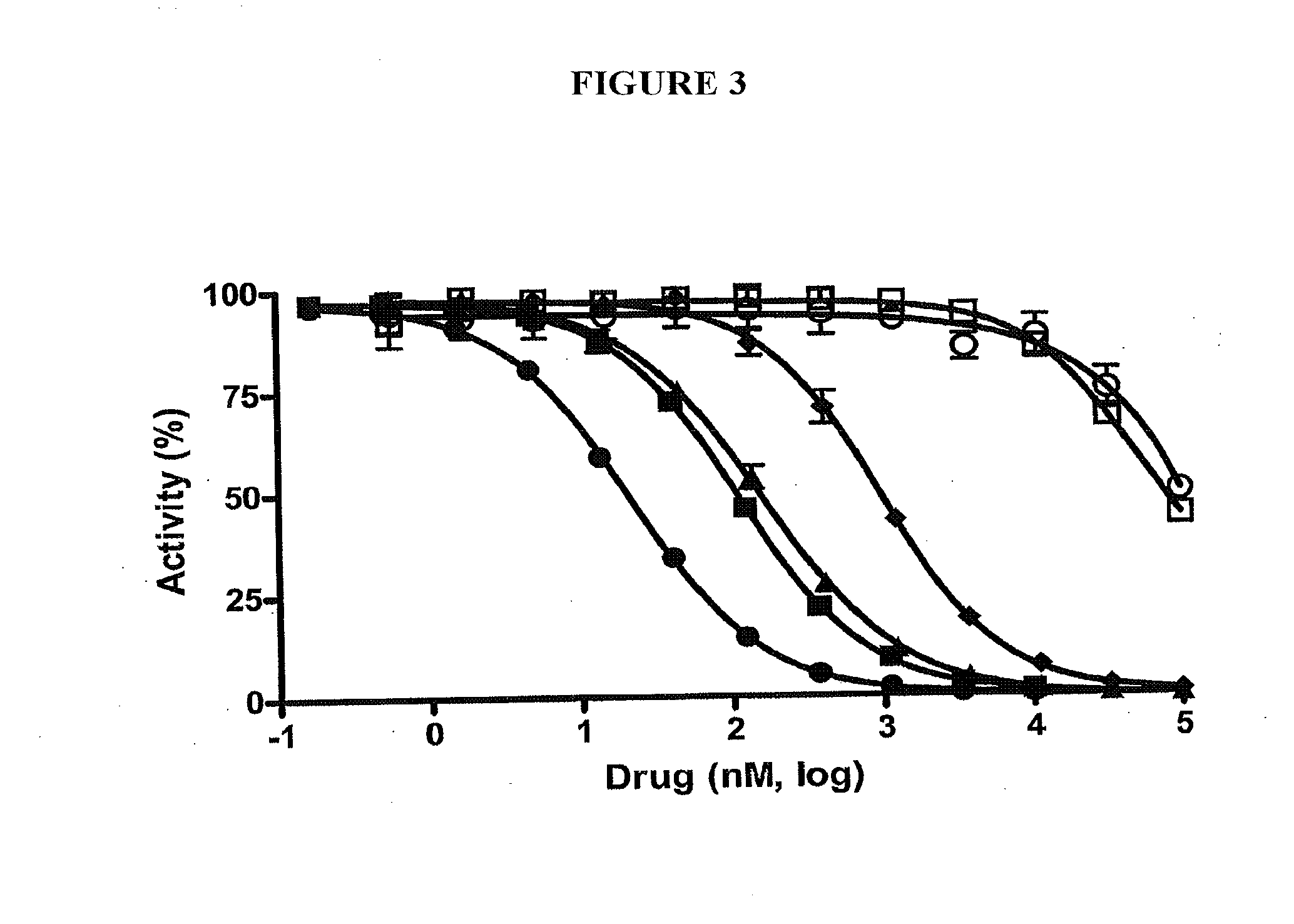
β-Glucuronidase Activity Assay
[0120]Expression and Purification of E. coli β-Glucuronidase. The full-length E. coli β-glucuronidase gene was obtained from bacterial genomic DNA and was cloned into the pET-28a expression plasmid (Novagen) with an N-terminal 6×-Histidine tag. BL21-DE3 competent cells were transformed with the expression plasmid and grown in the presence of kanamycin (25 ug / ml) in LB medium with vigorous shaking at 37° C. until an OD600 of 0.6 was attained. The expression was induced with the addition of 0.3 mM isopropyl-1-thio-D-galactopyranoside (IPTG) and further incubated at 37° C. for 4 hours. Cells were collected by centrifugation at 4500×g for 20 min at 4° C. Cell pellets were resuspended in Buffer A (20 mM Potassium Phosphate, pH 7.4, 25 mM Imidazole, 500 mM NaCl), along with PMSF (2 μL / mL from 100 mM stock) and 0.05 μL / mL of protease inhibitors containing 1 mg / mL of aprotinin and leupeptin. Resuspended cells were sonicated and centrifuged at 14,500×g for 30 mi...
example 2
Preparation of Analogs of BRITE-355252
[0123]General procedures for the preparation of analogs of BRITE-355252 All solvents and reagents were obtained from commercial sources and used without further purification unless otherwise stated. All reactions were performed in oven-dried glassware (either in RB flasks or 20 ml vials equipped with septa) under an atmosphere of nitrogen and the progress of reactions was monitored by thin-layer chromatography and LC-MS. Analytical thin-layer chromatography was performed on precoated 250 μm layer thickness silica gel 60 F254 plates (EMD Chemicals Inc.). Visualization was performed by ultraviolet light and / or by staining with phosphomolybdic acid (PMA) orp-anisaldehyde. All the silica gel chromatography purifications were carried out by using Combiflash® Rf (Teledyne Isco) and CombiFlash® Companion® (Teledyne Isco) either with EtOAc / hexane or MeOH / CH2Cl2 mixtures as the eluants. Melting points were measured on a MEL-TEMP® capillary melting point ...
example 3
Materials And Methods
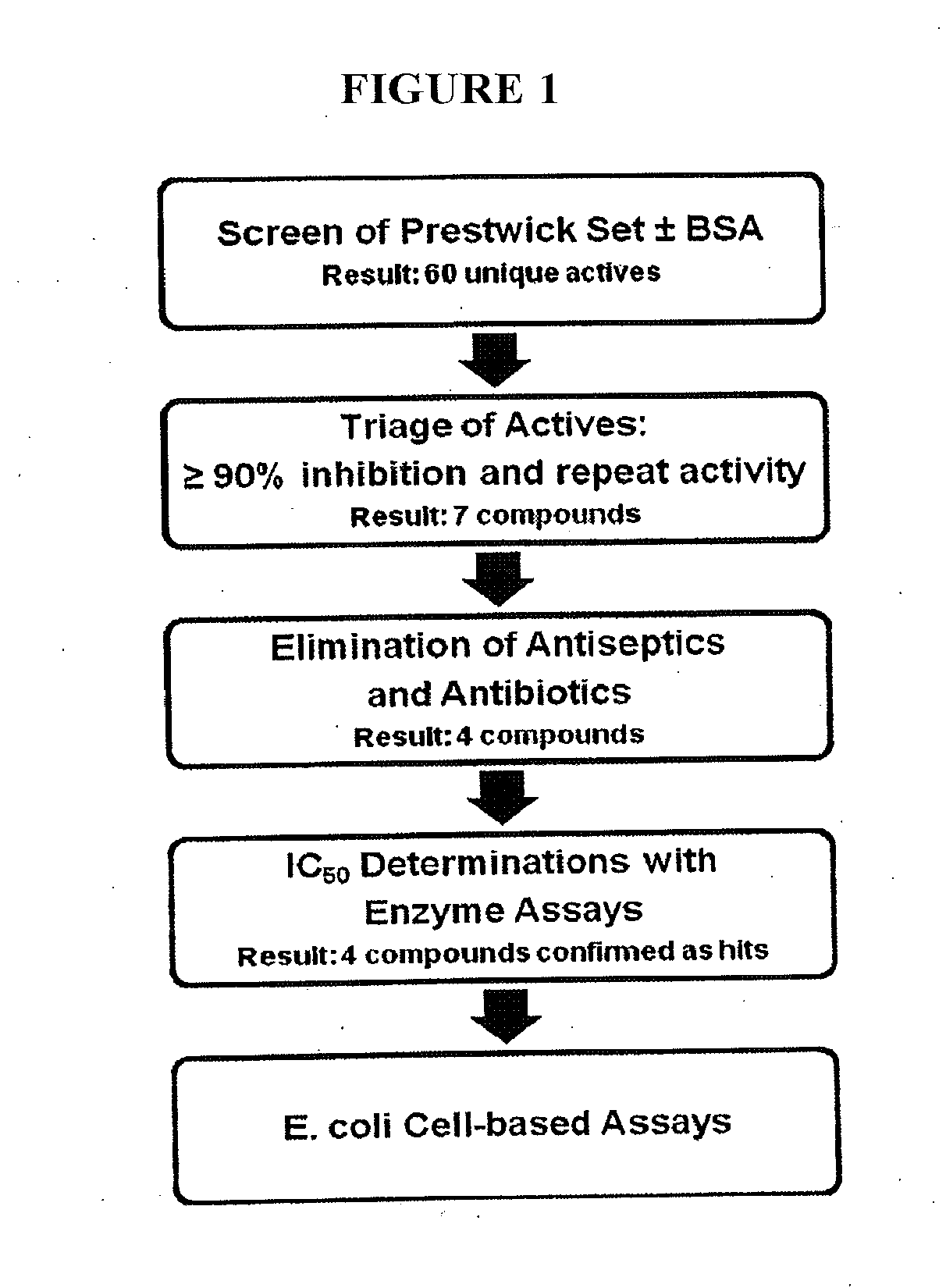
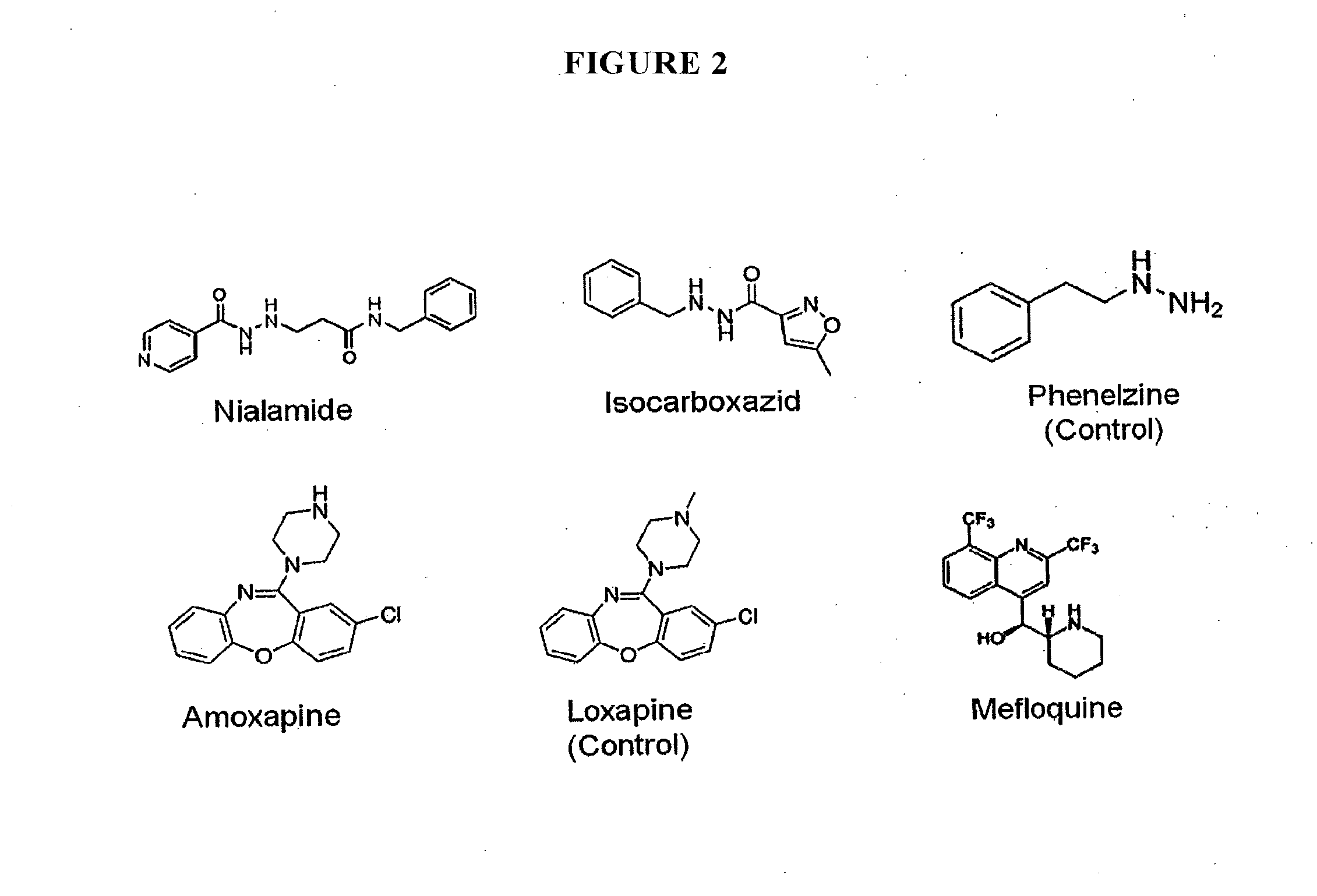
[0154]All common reagents such as HEPES, Triton X-100, carbenicillin, and dimethyl sulfoxide (DMSO) were reagent-grade quality and obtained from Thermo Fisher Scientific (Waltham, Mass.) or Sigma-Aldrich (St. Louis, Mo.). 4-methylumbelliferyl glucuronide (4MUG) was obtained from Sigma-Aldrich (St. Louis, Mo.). The solid black 96-well plates (cat#3915) for the assay and 96 well clear plates (cat#9017) for cytotoxicity assay were from Corning Incorporated (Corning, N.Y.). Falcon polypropylene plates (cat#1190) used for serial dilution of compounds were obtained from Becton Dickinson (Franklin Lake, N.J.). Amoxapine, nialamide, isocarboxazid and other compounds for follow-up studies were obtained from Sigma-Aldrich. The Prestwick Chemical Collection was obtained from Prestwick Chemical Company (Washington D.C.). E. coli DH5a (Zymo Research, Irvine, Calif.) was used for the cell-based assay. The expression and purification of GUS enzyme from E. coli carrying an expr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com