Serum antibody assay for determining protection from malaria, and pre-erythrocytic subunit vaccines
a malaria and antibody assay technology, applied in the field of serum antibody assay, can solve the problems of low-level immune response and minimal protection, cumbersome chmi, and high cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
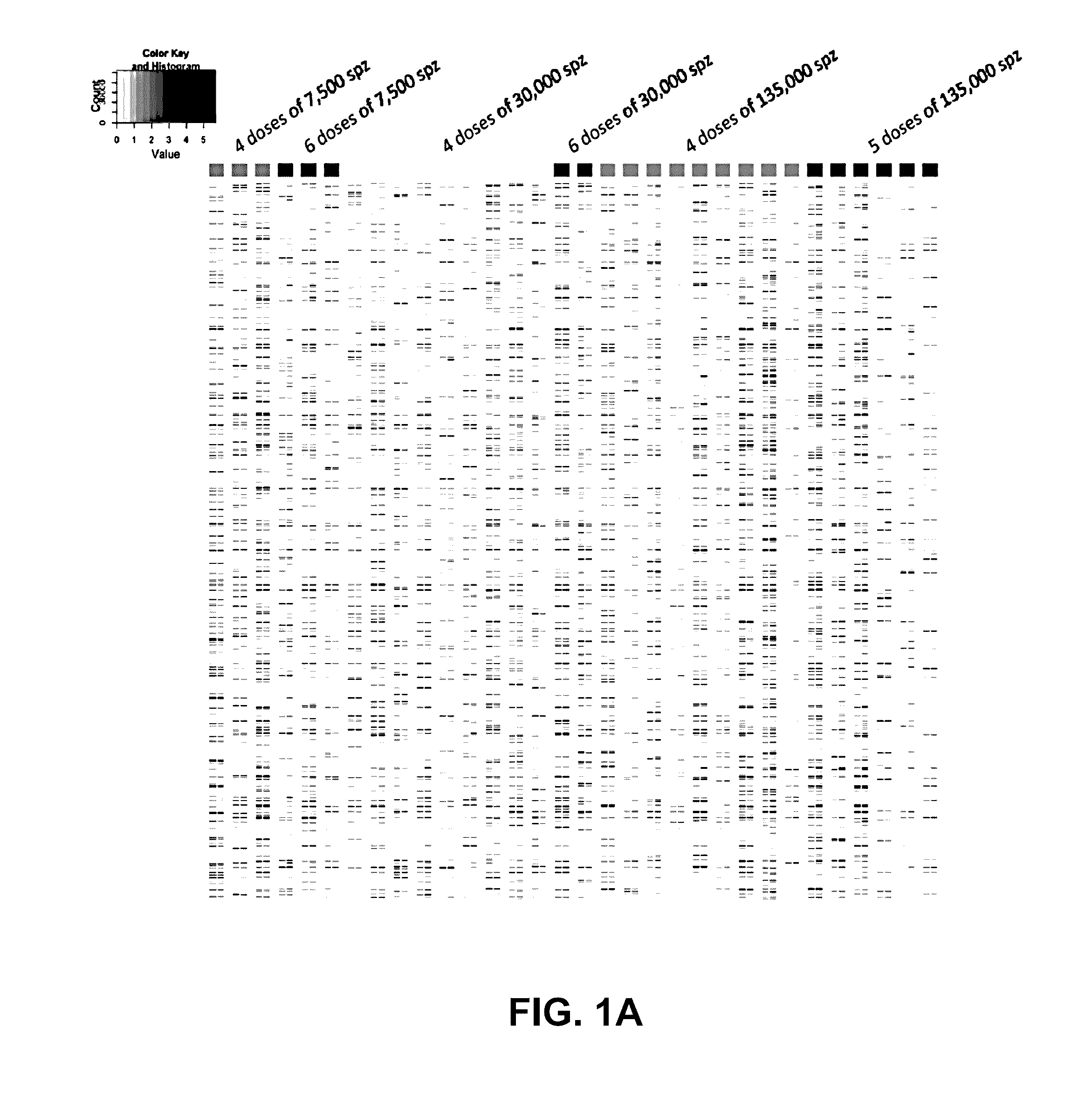
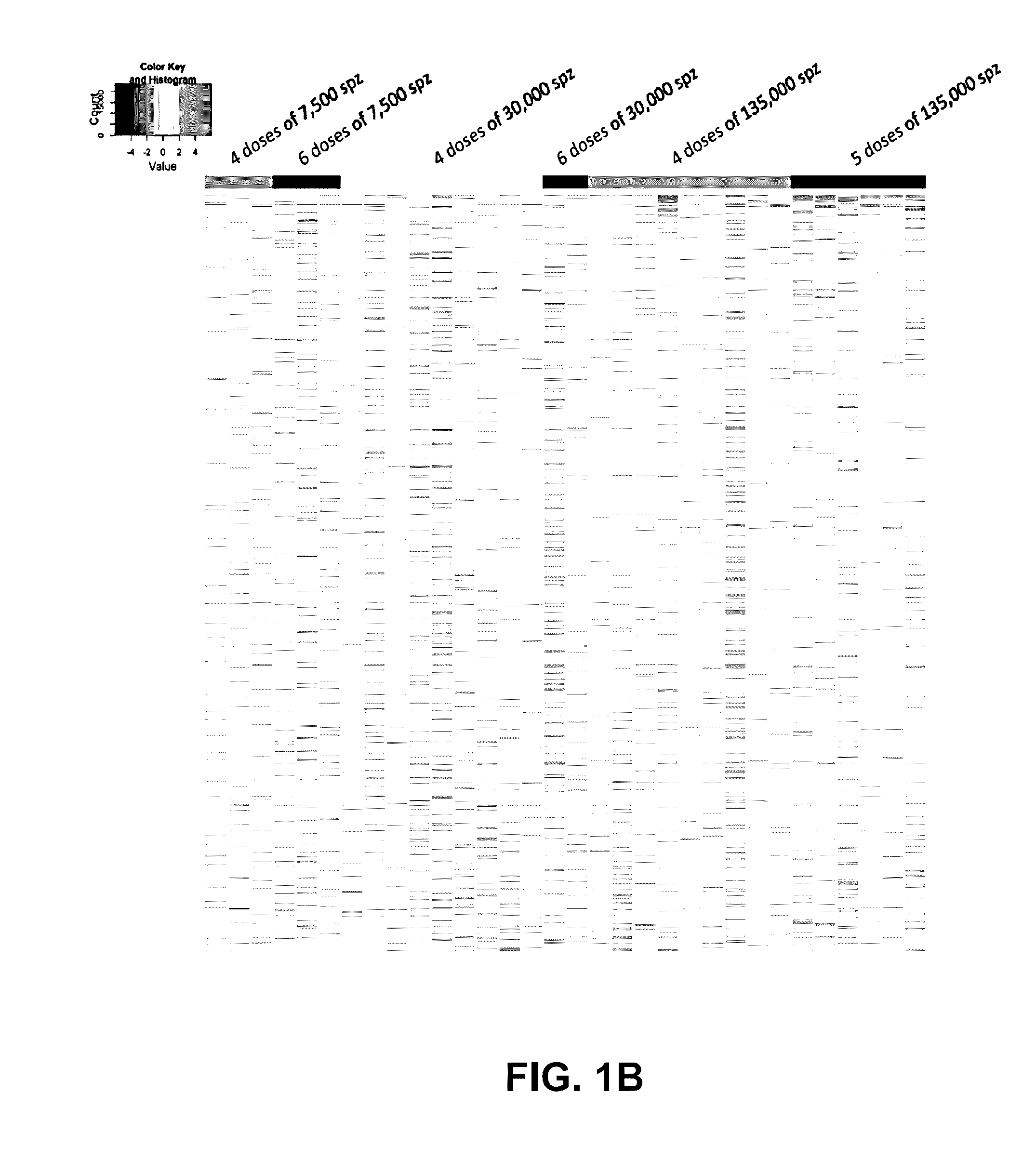
[0142]A clinical trial testing the immunogenicity and efficacy of aseptic, radiation-attenuated, purified, cryopreserved sporozoites used as the immunogen in a vaccine formulation (Sanaria® PfSPZ Vaccine, provided by Sanaria Inc.), and administered by intravenous injection, resulted in 13 individuals that were protected against controlled human malaria infection (CHMI) and 19 that were not protected (Table 4). In the group receiving the highest dosage (675,000 total PfSPZ), 6 out of 6 individuals were protected; and the group receiving the next lower dosage (540,000 total PfSPZ) had 6 out of 9 protected (Seder et al., Science 2013).
TABLE 4Protection efficacyTotalPfSPZ perdosage ofProtection %DoseDosesPfSPZ# volunteers# ProtectedProtection %Combined7500 (low)430000300%0%645000300%3000041200009111%9%(medium)6180000200%135000 (high)45400009667%80%567500066100%
[0143]The 5-dose (675000 SPZ) group in which all subjects were protected differed from other groups, e.g., these subjects receiv...
example 2
P. falciparum Proteome Microarray Chip Fabrication
[0144]P. falciparum (Pf) genes, representing 91% of the Pf proteome, were selected and cloned into the Escherichia coli (E. coli) expression vector pXT7. Included among these genes were those corresponding to SEQ ID NOs:1-10. Custom polymerase chain reaction (PCR) primers comprising 20-bp gene-specific sequences with 33-bp adapter sequences were used to amplify target amplicons from Pf genomic DNA. The adapter sequences, which flank the target amplicons, are homologous to the adapter sequences at the ends of the linearized T7 expression vector pXT7 into which they were cloned. The homology allows the amplified PCR products to be cloned into the expression vector by in vivo homologous recombination in competent DH5a cells. The resulting clone mixtures were then verified by PCR using sequence specific primers and subsequently sequenced. For proteins microarray chip fabrication, Pf proteins were expressed in E. coli-based cell-free in v...
example 3
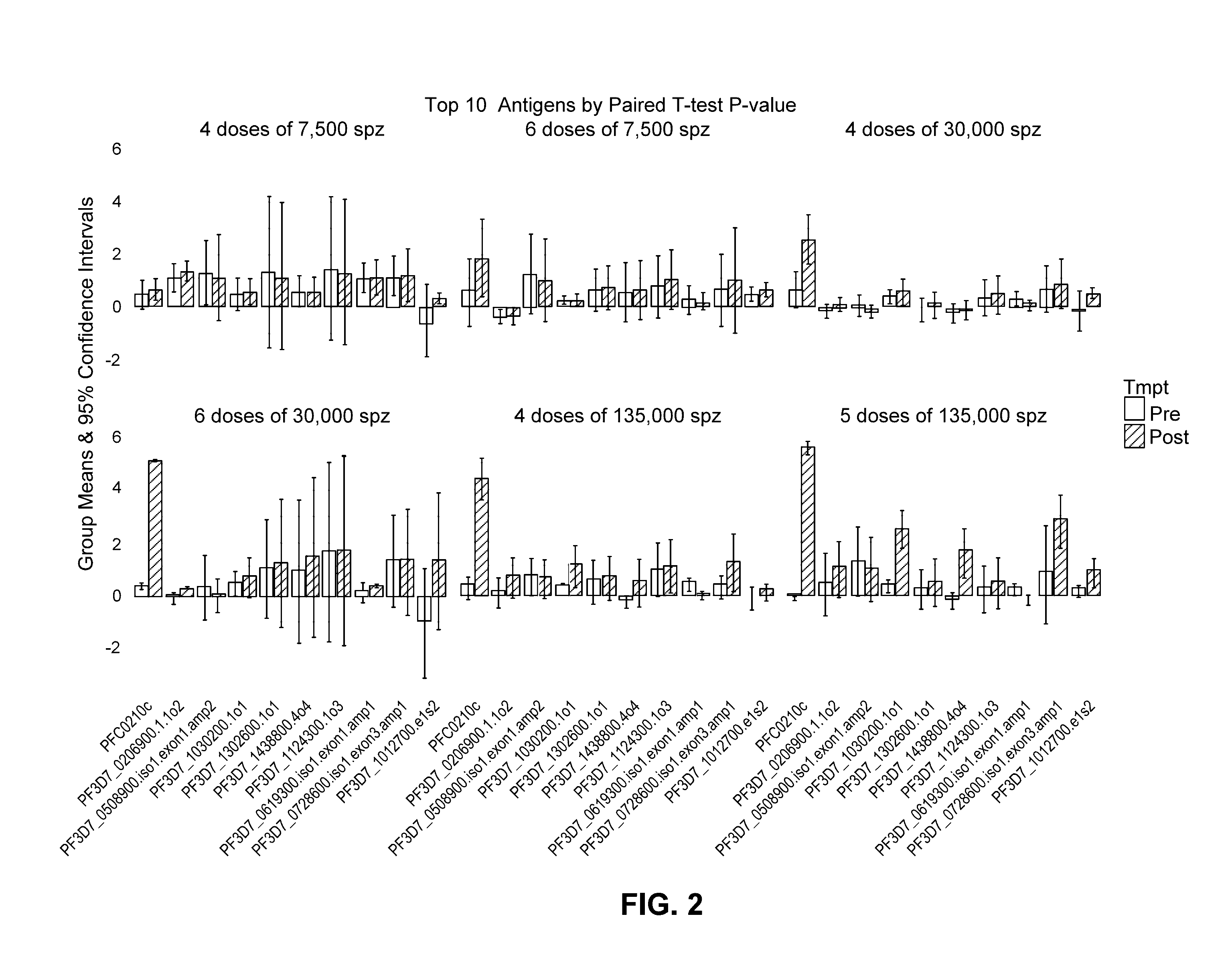
[0151]Among the 1,567 immunoreactive antigens, a subset of proteins was identified as useful for identifying of protected subjects, or being “Associated with Protection.” In addition to CSP and MSP5, pre-CHMI antibodies against antigens with high AUC were glycogen synthase kinase 3, or GSK3 (gene id: PF3D7_0312400; AUC: 0.94, BH P-value=0.004) and a conserved protein of unknown function (PF3D7_1030200; AUC: 0.88, BH P-value=0.036). These antigens and other top antigen are shown in FIG. 4.
[0152]For antibody Deltas (the difference between pre-immunization and pre-CHMI), we identified a leucine-rich repeat protein, called LRR9 (PF3D7_0906700; AUC: 0.90, BH P-value=0.003), and a protein described as double C2-like domain-containing protein, or DOC2 (PF3D7_1243900; AUC: 0.96, BH P-value=0.0005) with high AUC for protection. These antigens and other top antigens are shown in Table 6 and FIG. 5. As with the down-selected chip, the CSP was a top candidate with a pre-CHMI AUC of 0.93 (BH P-v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| OD | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
| heat maps | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap