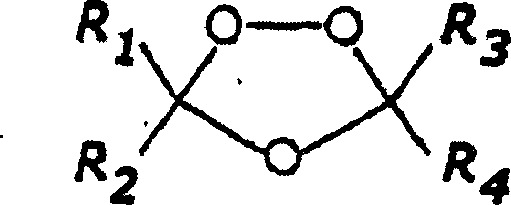
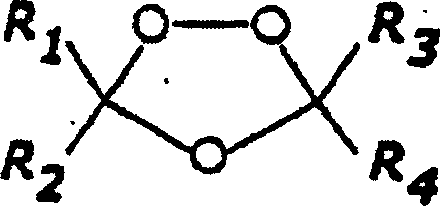
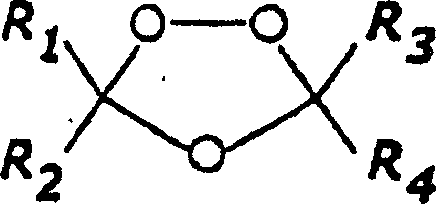
1,2,4-trioxolane antimalarials
A technology of trioxane and oxoxane, which can be used in anti-infective drugs, anti-tumor drugs, drug combinations and other directions, can solve problems such as neurotoxicity, recurrence, and metabolic instability, and achieve low neurotoxicity, simple structure, and ease of use. and cheap synthetic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] General method for the preparation of 1,2,4-trioxolane
[0144] Synthesis of O-methyl 2-adamantanone oxime (representative method)
[0145] To a solution of 2-adamantanone (4.51 g, 30 mmol) in methanol (30 ml) was added pyridine (4.5 ml) and methoxylamine hydrochloride (3.76 g, 45.0 mmol). The reaction mixture was stirred at room temperature for 48 h, concentrated in vacuo, and washed with CH 2 Cl 2 (50ml) and water (50ml) to dilute. The organic layer was separated and washed with CH 2 Cl 2 (30ml) to extract the aqueous layer. The combined organic extracts were washed with 1M HCl (30ml×2) and saturated aqueous NaCl (30ml), and washed with MgSO 4 dry. Evaporation in vacuo gave O-methyl 2-adamantanone oxime (4.77 g, 89%) as a colorless solid. mp70-71℃; 1 H NMR (300MHz, CDCl 3 ) δ 1.60-2.10 (m, 12H), 2.54 (s, 1H), 3.47 (s, 1H), 3.82 (s, 3H).
[0146] References: Corey, E.J.; Niimura, K.; Konishi, Y.; Hashimoto, S.; Hamada, Y.A New Synthetic Route to Pr...
Embodiment 2
[0707] Antimalarial activity of OZ01-OZ270
[0708] Each trioxolane was screened against the chloroquine-resistant K1 strain and the chloroquine-sensitive NF54 strain of Plasmodium falciparum in vitro. In a single-dose STI in vivo screen, Moro genus infected with the ANKA strain of P. berghei (P. SPF or NMRI mice (groups of three mice). Trioxolane was administered subcutaneously and orally in a single 10 mg / kg dose. Trioxolane was also administered as a single 10 mg / kg dose in a standard suspension vehicle (SSV). SSV consisted of 0.5% w / v CMC, 0.5% v / v benzyl alcohol, 0.4% v / v Tween 80 and 0.9% w / v sodium chloride in water. Antimalarial activity was determined by the percent reduction in parasitemia and survival time at day 3 post-infection compared to untreated controls. Survival to day 30 post-infection was considered cured. Regarding the comparative analysis, Table 1 below shows the data for OZ01-OZ270 and the control group, fenozan, artemisinin, artethe...
Embodiment 3
[0975] Activity of trioxane against Plasmodium berghei
[0976] As shown in Table 2 below, the trioxane compounds have significant in vivo antimalarial activity against Plasmodium berghei as determined in a 4-day Peters assay. According to oral ED 50 / ED 90 value, OZ23 is the compound with the strongest oral activity among all trioxolanes and control compounds.
[0977] Table 2
[0978] Compound Plasmodium berghei Plasmodium yoelii (P.yoelii)
[0979] ED 50 / ED 90 (mg / kg) ED 50 / ED90 (mg / kg)
[0980] Oral Subcutaneous Subcutaneous
[0981] OZ03 1.0 / 2.7 0.6 / 1.1 1.6 / 3.1
[0982] OZ05 1.5 / 6.1 0.6 / 1.0 1.4 / 2.5
[0983] OZ10 1.3 / 3.0 0.5 / 0.9 1 / 2.2
[0984] OZ11 1.8 / 3.1 0.5 / 0.9 1.0 / 2.1
[0985] OZ12 6.8 / 10.5 0.8 / 1.4 1.1 / 2.3
[0986] OZ15 2.9 / 6.3 0.7 / 1.1 1.4 / 3.6
[0987] OZ23 0.9 / 2.0 0.5 / 0.9 0.7
[0988] OZ25 1.2 / 3.7 2.2 / 3.7 2.7 / 4.3
[0989] OZ27 1.3 / 2.6 0.2 / 0.9 4.4 / 10.2
[0990] OZ32 2.6 / 5.1 0.6 / 1.3 1.2 / 3.5
[0991] Fen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com