Compositions and Methods for Improving Rebaudioside M Solubility
a technology of rebaudioside and solubility, applied in the field of compositions and methods for improving rebaudioside m solubility, can solve the problems of poor aqueous solubility and dissolution quality of crystalline rebaudioside m in beverage formulations, and achieve the effect of reducing precipitation and improving aqueous solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
l Elucidation of Rebaudioside M
[0643]HRMS: HRMS (High Resolution Mass Spectrum) data was generated with a Waters Premier Quadrupole Time-of-Flight (Q-TOF) mass spectrometer equipped with an electrospray ionization source operated in the positive-ion mode. Samples were diluted and eluted with a gradient of 2:2:1 methanol:acetonitrile:water and introduced 50 μL via infusion using the onboard syringe pump
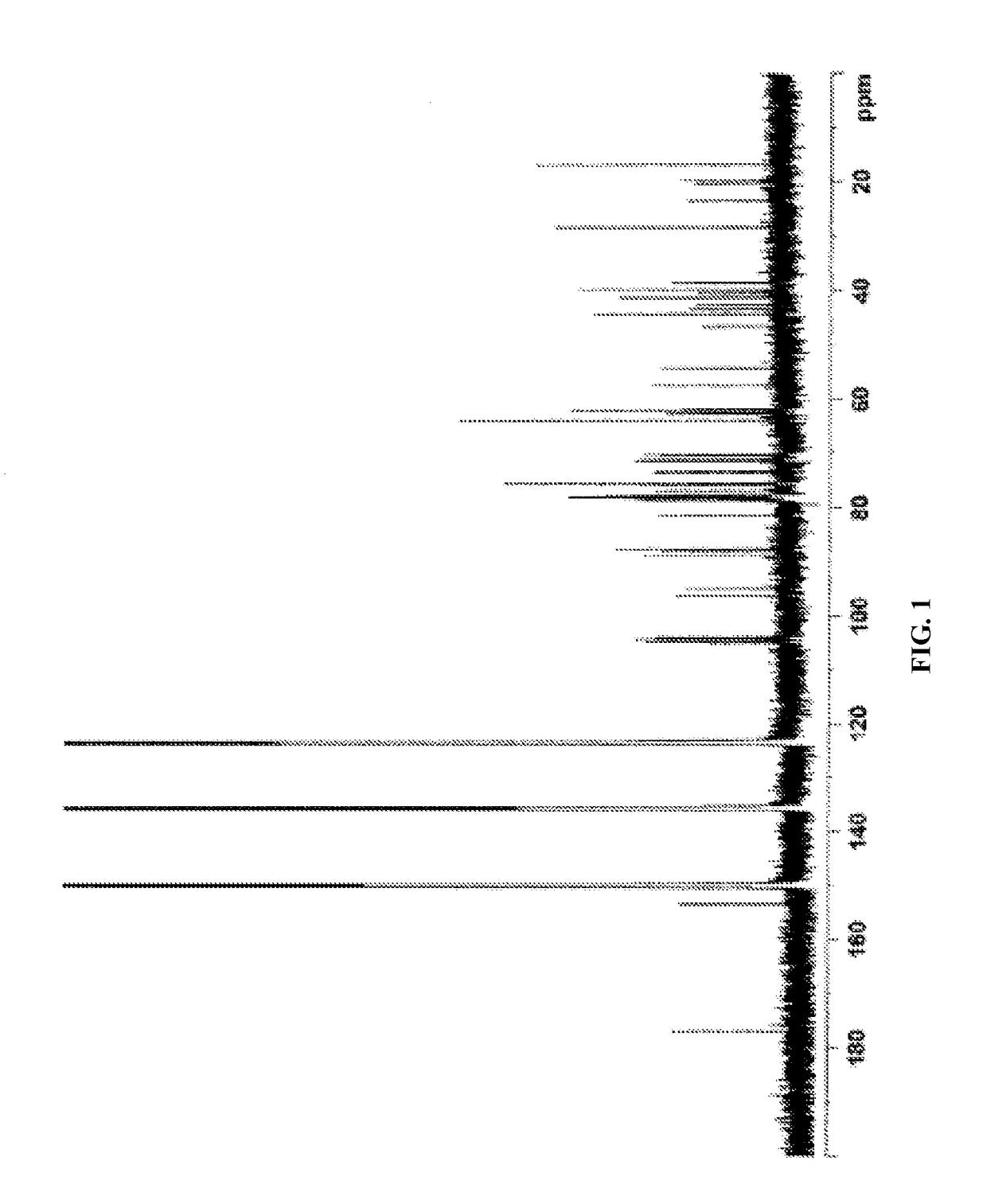
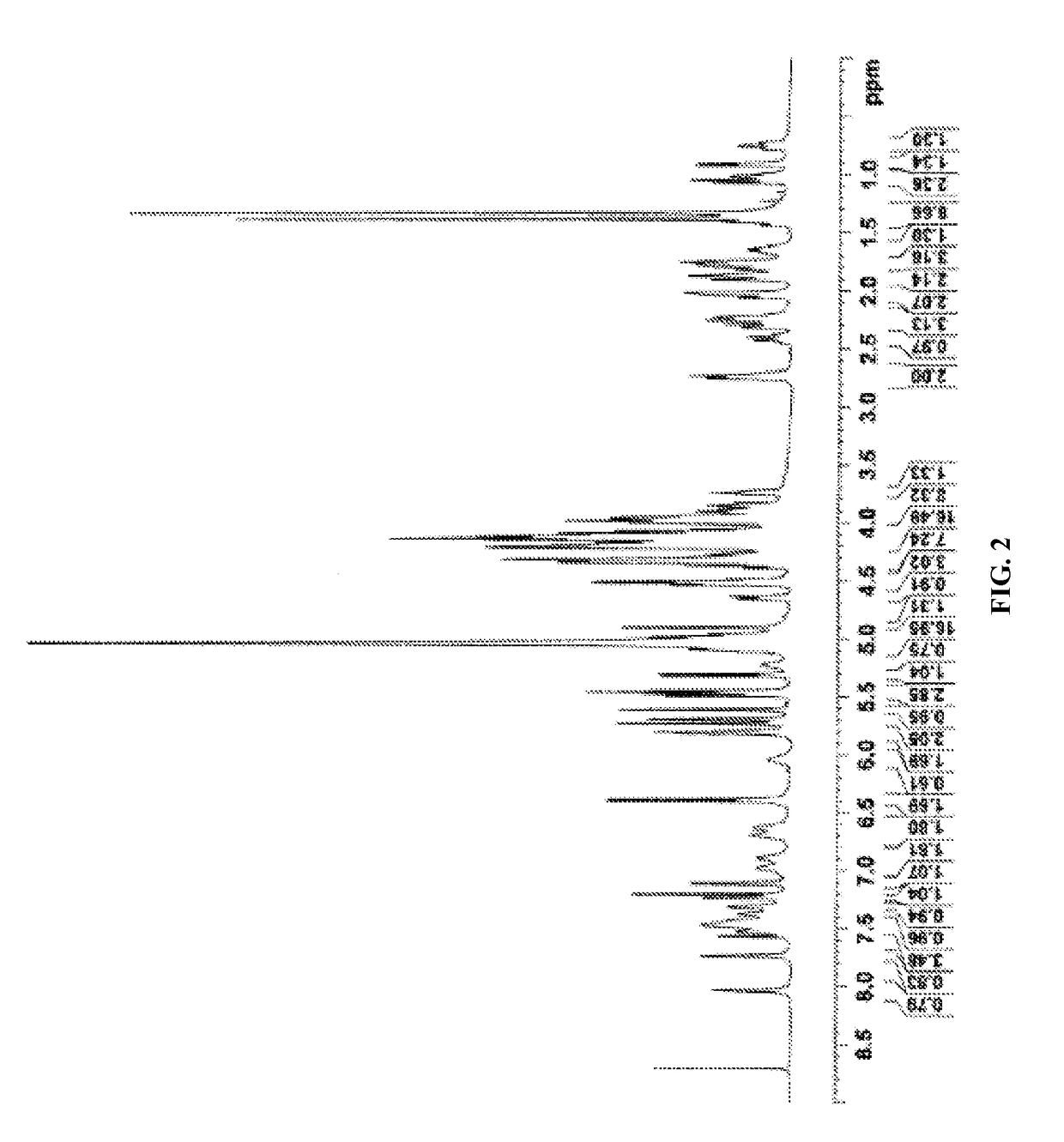
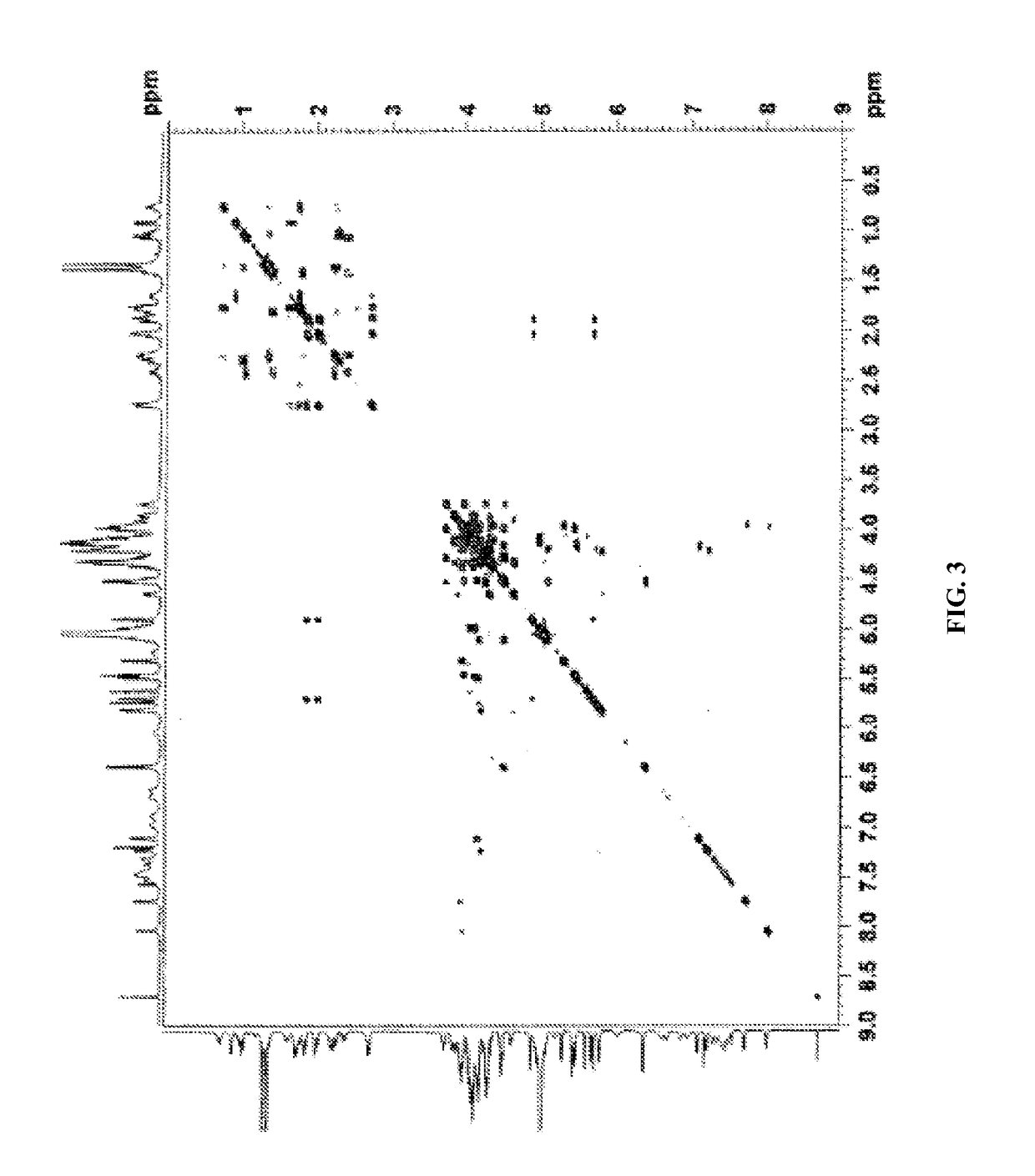
[0644]NMR: The sample was dissolved in deuterated pyridine (C5D5N) and NMR spectra were acquired on Varian Unity Plus 600 MHz instruments using standard pulse sequences. The chemical shifts are given in δ (ppm), and coupling constants are reported in Hz.
[0645]The complete 1H and 13C NMR spectral assignments for the diterpene glycoside rebaudioside M determined on the basis of 1D (1H and 13C) and 2D (COSY, HMQC and HMBC) NMR as well as high resolution mass spectroscopic data:
[0646]Discussion
[0647]The molecular formula was deduced as C56H90O33 on the basis of its positive high resolution...
example 3
on of Disordered Crystalline Rebaudioside M Composition
[0653]A 100 g sample containing rebaudioside D (11.7%), rebaudioside M (84.2%), rebaudioside A (1.8%), stevioside (0.1%), rebaudioside B (1.2%) (referred to herein as “RebM80”)—all percentages being on a percent dry weight basis—and having water solubility of 0.1% (determined visually at room temperature with stirring for 5 minutes), was mixed with 900 g of water and incubated in airtight pressure vessel placed in a thermostatted oil bath. The temperature was increased at 2° C. per minute to 121° C. The mixture was maintained at 121° C. for 10 minutes and then the temperature was decreased to 100° C. at 2° C. per minute to give a concentrated solution of RebM80.
[0654]1,000 g of the concentrated solution was constantly maintained at 100° C. while being fed via insulated piping to YC-015 laboratory spray drier (Shanghai Pilotech Instrument & Equipment Co. Ltd., China) operating at 175° C. inlet and 100° C. outlet temperature. 98 g...
example 4
on of Spray-Dried Rebaudioside M Compositions
[0675]A 10 g sample of RebM80 having water solubility of 0.1% was mixed with 200 mL of water in an open flask and placed in oil bath. The mixture was heated to 100° C. over 1˜2 h to give a concentrated solution of rebaudioside M.
[0676]About 200 g of the concentrated solution was constantly maintained at ˜90-100° C. while being fed via insulated piping to Mini-spray drier ADL310 (YAMATO) operating at ˜140° C. inlet and ˜80° C. outlet temperature. 8 g (80% yield) of disordered crystalline product was obtained.
[0677]The blends in Table 4 were prepared using a similar procedure, varying in the addition of SG95RA50 or NSF-02 and the amount of water used. SG95RA50 was obtained from Cargill. NSF-02 was obtained from Pure Circle.
TABLE 4Experi-WaterWatermentSampleAmounts(mL)Yield g (%)Content4aRebM80 / 33.25 g700 26 g (74%)3.52%SG95RA50RebM80,1.75 gSG95RA504bRebM80 / 31.5 g70028.3 g (81%)5.82%SG95RA50RebM80,3.5 gSG95RA504cRebM80 / 31.5 g70029.1 g (83%)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com