Compositions and methods for providing hematopoietic function without HLA matching
a technology of hematopoietic function and composition, applied in the field of compositions and methods for providing hematopoietic function to human, can solve the problems of organ transplant rejection, increase morbidity and mortality, and significant infection risk, and achieve the effects of increasing the number of scid repopulation cells, increasing the number of hematopoietic stem cells, and increasing the number of cd34+ cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
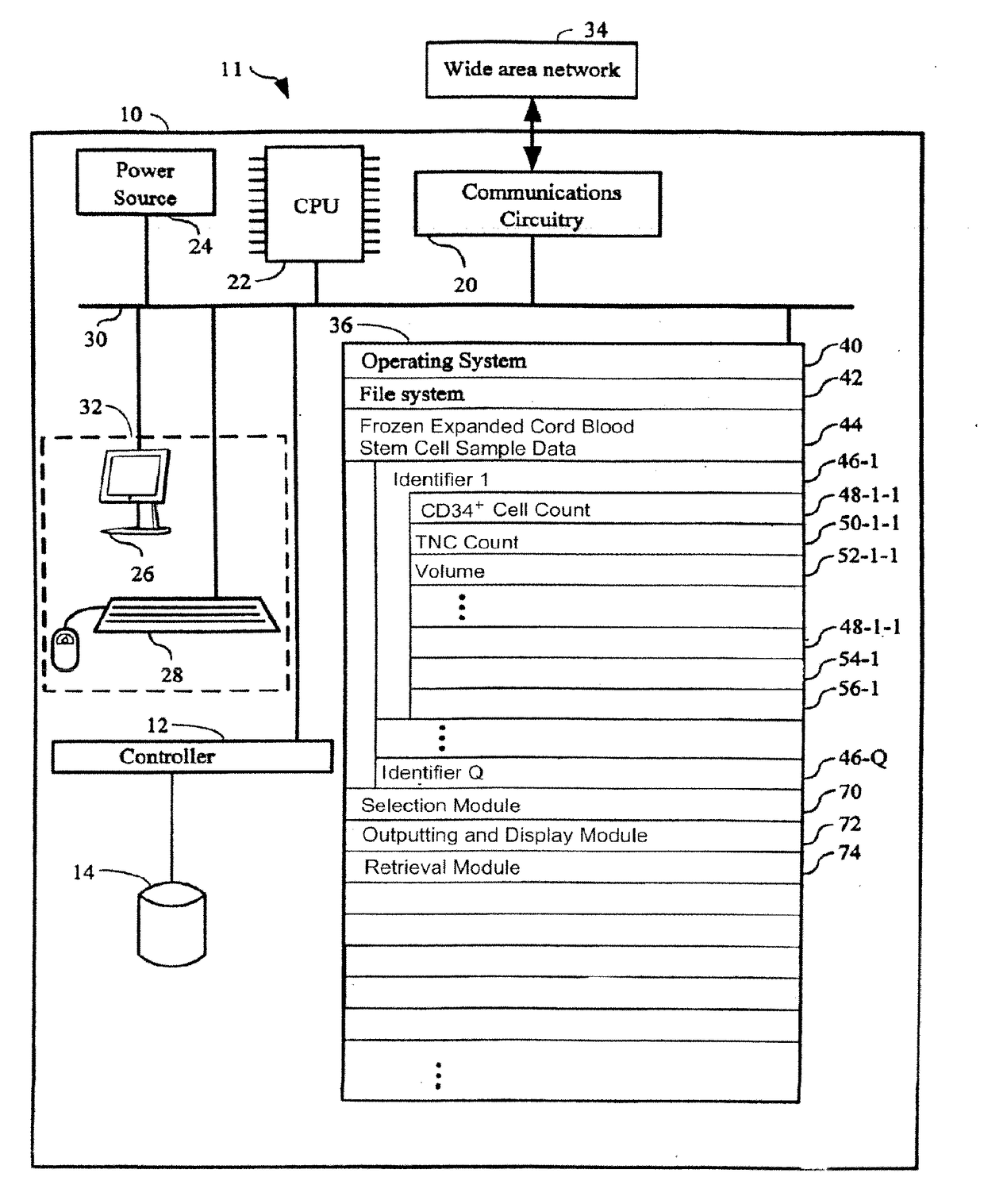
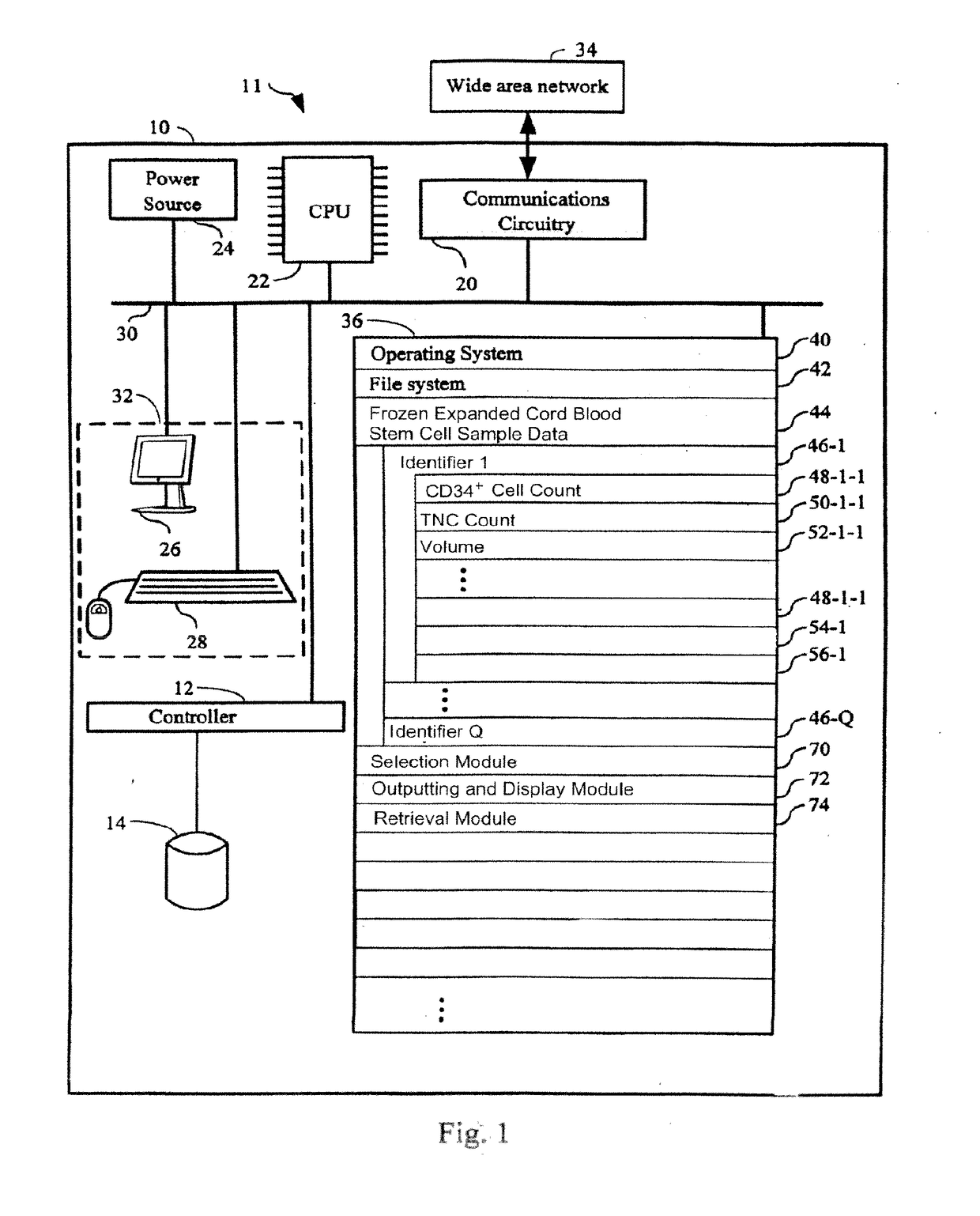
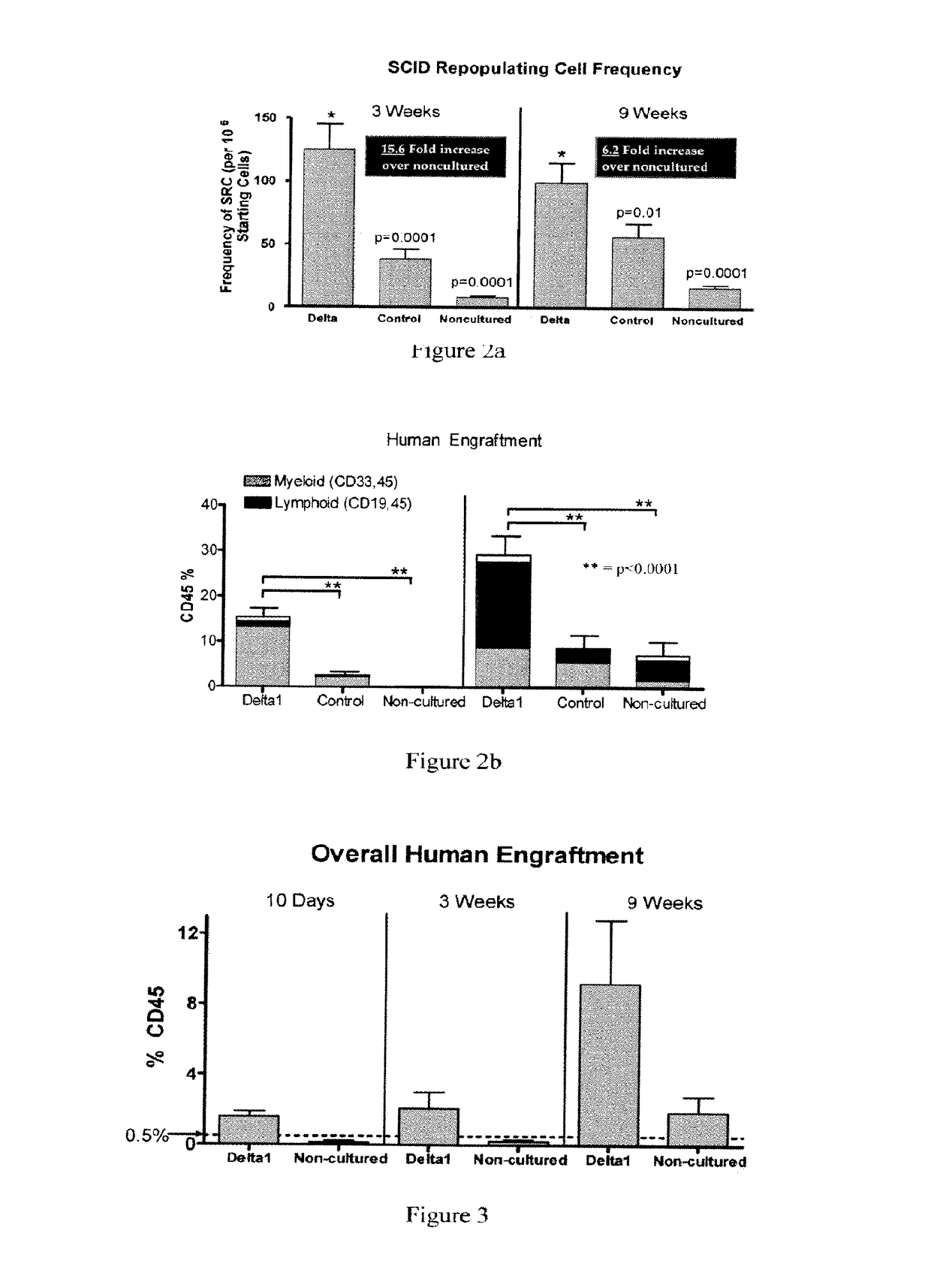
[0060]The present invention provides a method for providing hematopoietic function to a human patient in need thereof by administering an expanded human cord blood stem cell sample to the patient, wherein said administering is done without matching the HLA-type of the expanded human cord blood stem cell sample to the HLA type of the patient. By “without matching the HLA-type,” what is meant is that no steps are taken to have any of the HLA antigens or alleles match between the patient and the sample. In one embodiment, the expanded human cord blood stem cells can differentiate into cells of the myeloid lineage. In another embodiment, the expanded human cord blood stem cell can differentiate into cells of the lymphoid lineage. The selection of the Expanded CB Stem Cells to be administered to the patient is done without taking into account whether the patient to whom the Expanded CB Stem Cells will be administered matches or mismatches the Expanded CB Stem Cells at any of the HLA anti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com