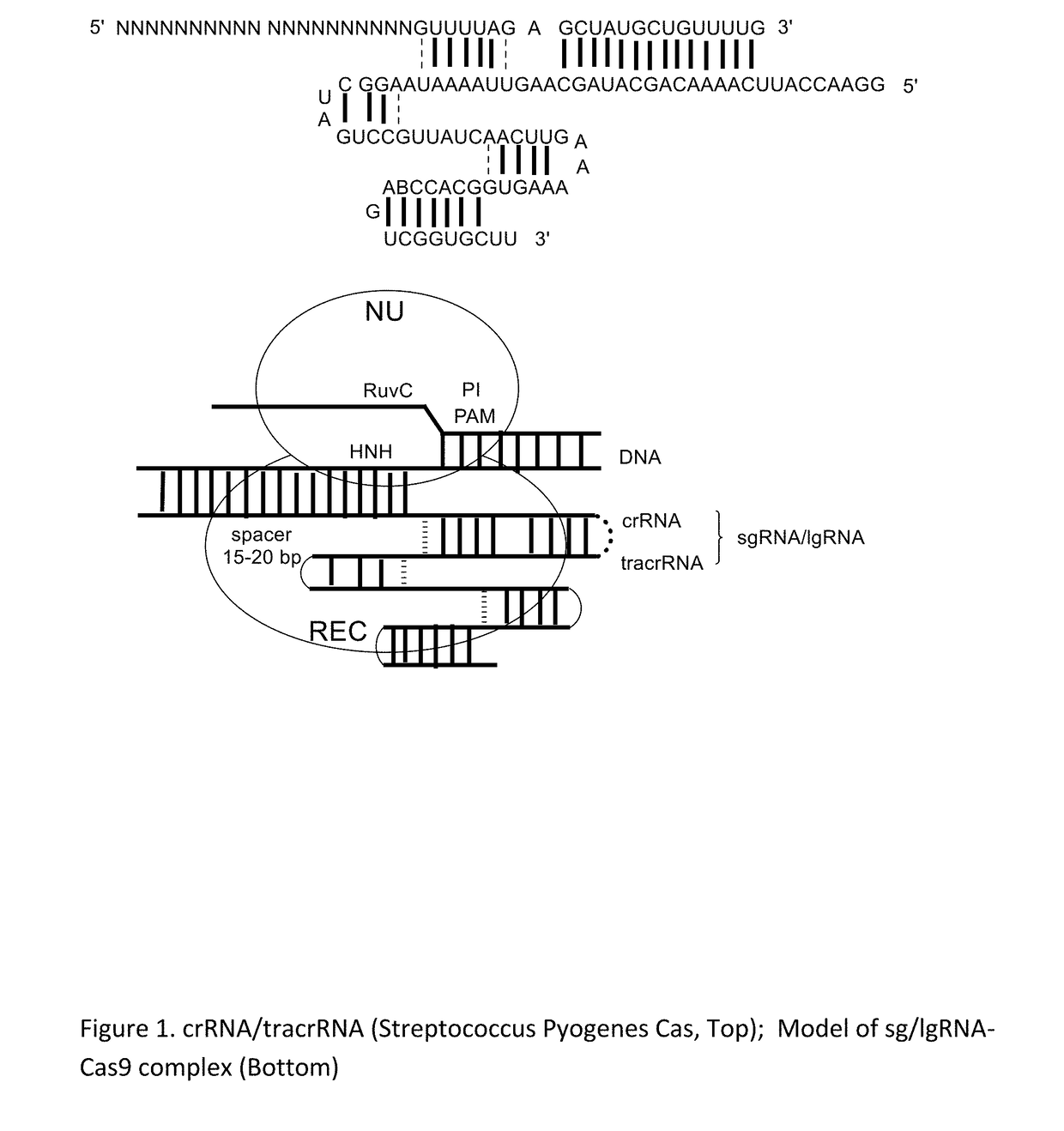
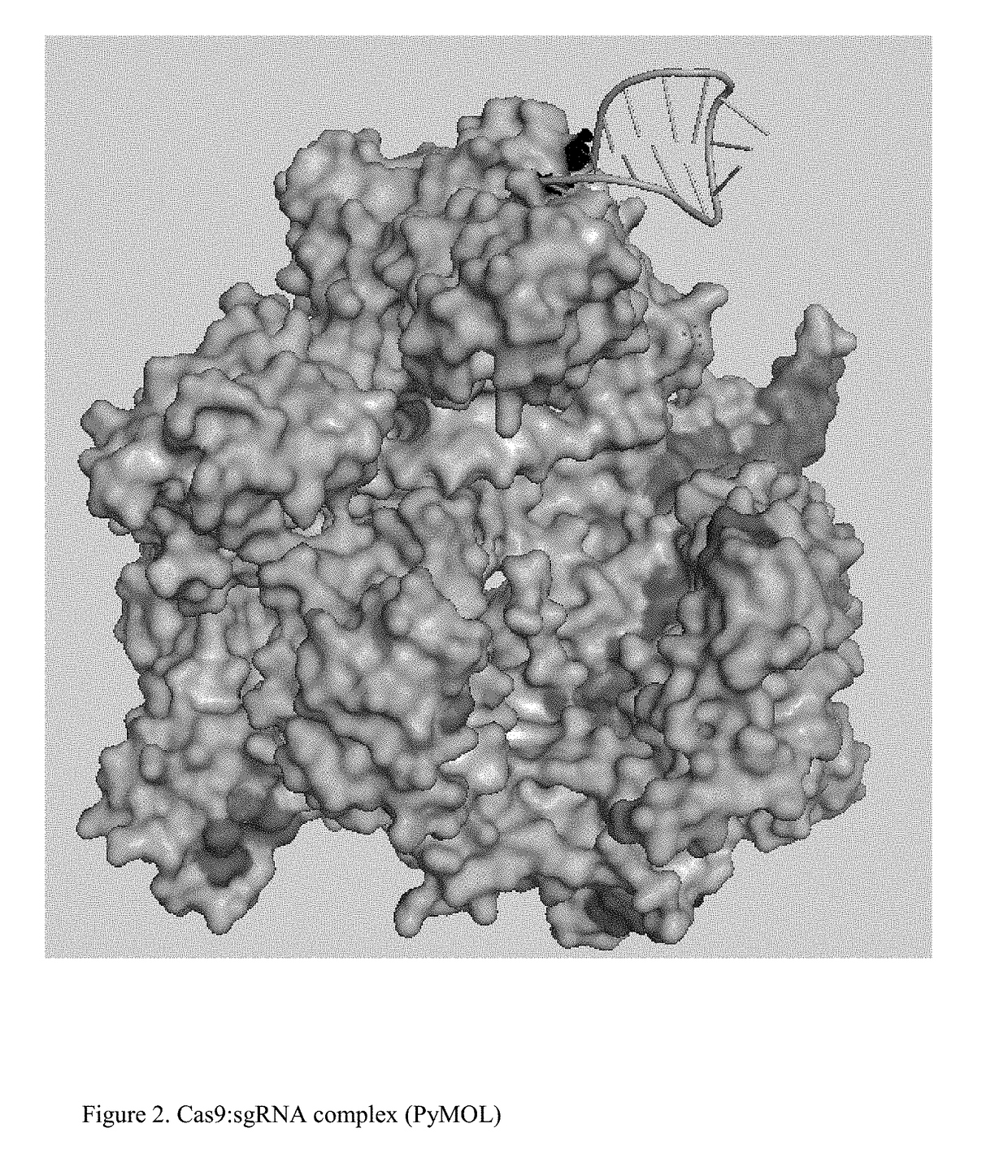
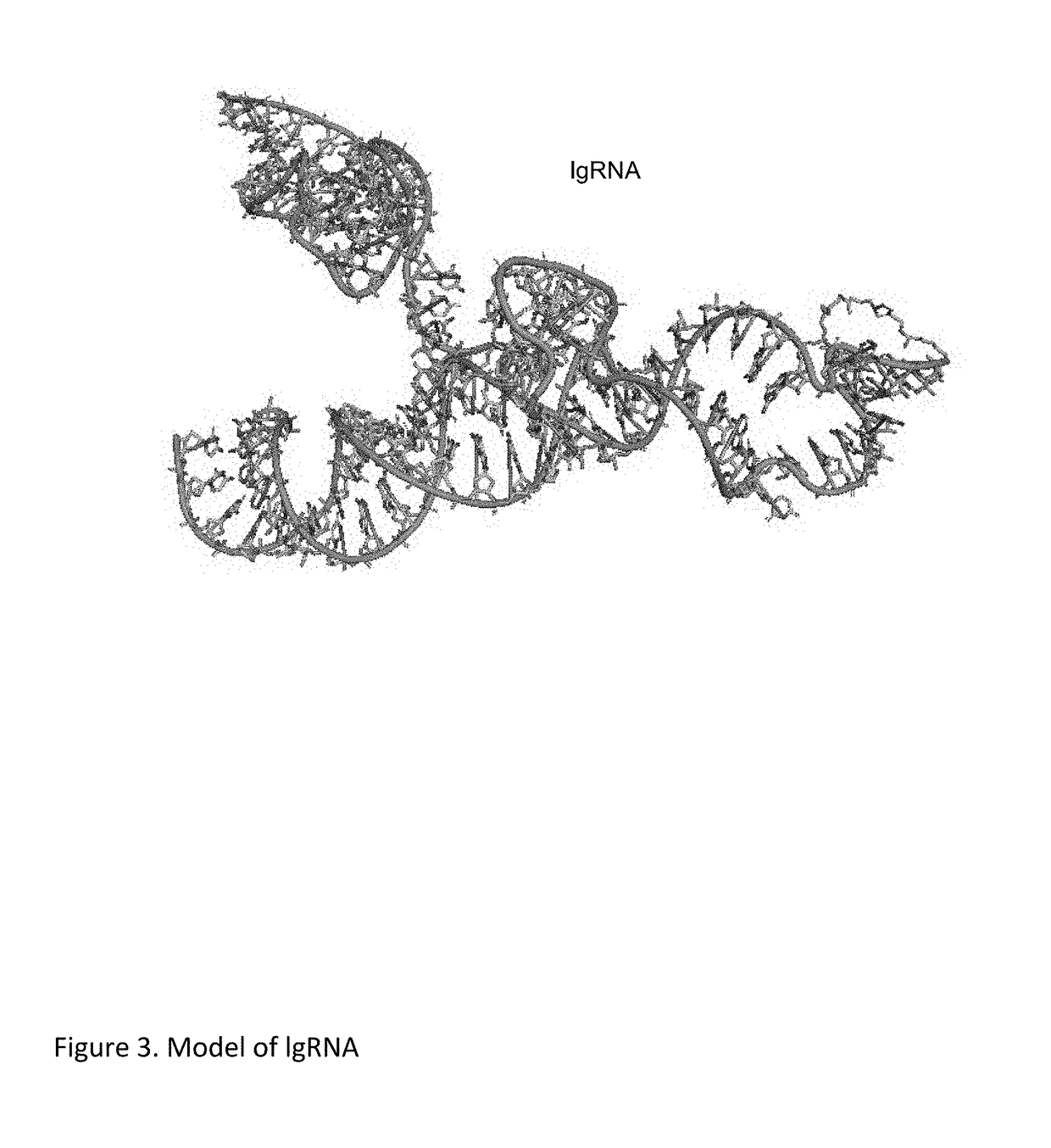
Chemically Ligated RNAs for CRISPR/Cas9-lgRNA Complexes as Antiviral Therapeutic Agents
a technology of lgrna and lgrna, which is applied in the direction of biochemistry apparatus and processes, peptide/protein ingredients, enzyme stabilisation, etc., can solve the problems of hsv, hbv, and hsv afflicting enormous suffering, life loss, and increased hes burden, so as to optimize the effect of target cleavag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0129]Compound 4
[0130]Compound 2 is prepared essentially according to a reported procedure in 4 steps (Santner, T. et al. Bioconjugate Chem. 2014, 25, 188-195). Compound 1 is treated with 2-azidoethanol in dimethylacetamide at 120 ° C. at the presence of BF3.OEt2, and the resulting 2′-azido nucleoside is tritylated (DMTrCl, in pyridine, RT), and attached to an amino-functionalized support.
[0131]To compound 2 (0.99 mmol) in THF / H2O (2:1) (18 mL) is then added trimethylphosphine (1.5 mL, 1.5 mmol). Reaction is shaken at room temperature for 8 h and washed thoroughly with THF / H2O (2:1). Dioxane / H2O (1:1) (20 mL) and NaHCO3 (185 mg, 2.2 mmol) are added. The reaction mixture is cooled down to 0° C., and Fmoc-OSu (415 mg, 1.23 mmol) in dioxane (2 mL) is added. The reaction is shaked for 15 min at 0° C., and then washed with water and then THF (3×20 mL) to give compound 4.
example 2
7
[0132]
[0133]6-Benzoyl-5′-O-DMTr-adenosine 5 (1.24 g, 1.84 mmole) is dissolved in THF (40 mL) and sodium hydride (60% dispersion in mineral oil, 0.184 g, 4.6 mmole) is added in portions at 0° C. The reaction mixture is warmed up to room temperature and stirred for 15 min. The reaction is then cooled to 0° C., and propargyl bromide (80% in toluene, 0.44 mL, 3.98 mmole) is added. The reaction is then stirred under reflux for 12 h. Saturated aqueous sodium bicarbonate (10 mL) is added, and volatiles are removed in vacuo. The resulting residue is dissolved in DCM, and washed with water and saturated brine. The organic layer is collected, dried over anhydrous sodium sulfate, and concentrated till dryness in vacuo. The resulting residue is purified by silica-gel chromatography (eluent: 97:3, DCM : MeOH, 0.5% pyridine) to provide compound 6.
[0134]To a solution of compound 6 (0.30 g, 0.42 mmol) in anhydrous dichloromethane (5 mL) and diisopropylethylamine (0.15 mL, 0.83 mmol), under nitroge...
example 3
[0135]
[0136]ON-11 is prepared using 2′-TBS protected RNA phosphoramidite monomers with t-butylphenoxyacetyl protection of the A, G and C nucleobases and unprotected uracil. 0.3 M Benzylthiotetrazole in acetonitrile (Link Technologies) is used as the coupling agent, t-butylphenoxyacetic anhydride as the capping agent and 0.1 M iodine as the oxidizing agent. Oligonucleotide synthesis is carried out on an Applied Biosystems 394 automated DNA / RNA synthesizer using the standard 1.0 μmole RNA phosphoramidite cycle. Compound 4 (20 mg) is packed into a twist column. All β-cyanoethyl phosphoramidite monomers are dissolved in anhydrous acetonitrile to a concentration of 0.1 M immediately prior to use. Stepwise coupling efficiencies are determined by automated trityl cation conductivity monitoring and in all cases are >96.5%.
[0137]Fmoc is then cleaved by treatment with 20% piperidine in DMF. The resulting 3′-end aminoethyl oligonucleotide is then treated with NHS ester of 6-azido caproic acid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com