Compositions and methods for nucleic acid and/or protein payload delivery
a nucleic acid and/or protein technology, applied in the direction of powder delivery, dna/rna fragmentation, macromolecular non-active ingredients, etc., can solve the problems of low cellular transfection level of nanoparticle-based payload delivery technology and limited transfection effectiveness, so as to induce proliferation and/or bias differentiation of target cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
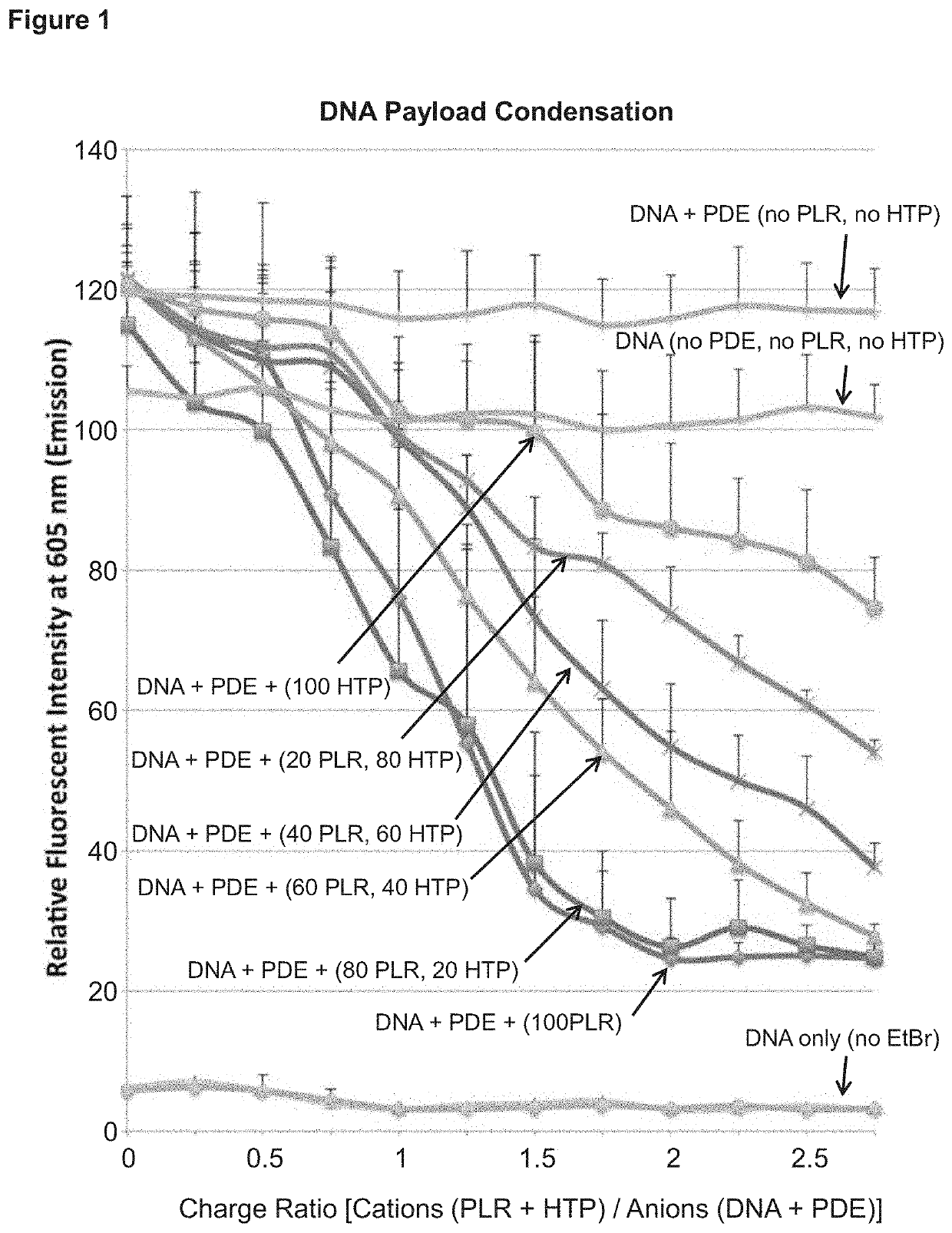
[0376]To determine core formulation parameters, fluorescence spectroscopy was used to monitor nucleic acid condensation (for double stranded DNA payloads). The emission spectra of intercalating Ethidium bromide (EtBr) was measured after the addition of condensing agents at increasing charge ratios. De-intercalation of ethidium bromide caused by polymer induced nucleic acid condensation results in a drop in fluorescent signal. This is because ethidium bromide exhibits a much higher quantum fluorescent yield in the DNA-bound state, than unbound state. The results are depicted in FIG. 1.
[0377]FIG. 1 depicts results from a fluorimetric assay testing various parameters (e.g., cation: anion charge ratio) for condensation of nucleic acid payloads. The result showed, e.g., that a charge ratio of 2 works well for the condensation of plasmids encoding Cas9 and guide RNA molecules. 100 μl of Anionic solution was added to each well: 100 ng / μl DNA, 80 ng / μl poly(D-Glutamic Acid) (PDE), 0.5 ng / μl...
example 2
e Synthesis Method
[0378]Procedures were performed within a sterile, dust free environment (BSL-II hood). Gastight syringes were sterilized with 70% ethanol before rinsing 3 times with filtered nuclease free water, and were stored at 4° C. before use. Surfaces were treated with RNAse inhibitor prior to use.
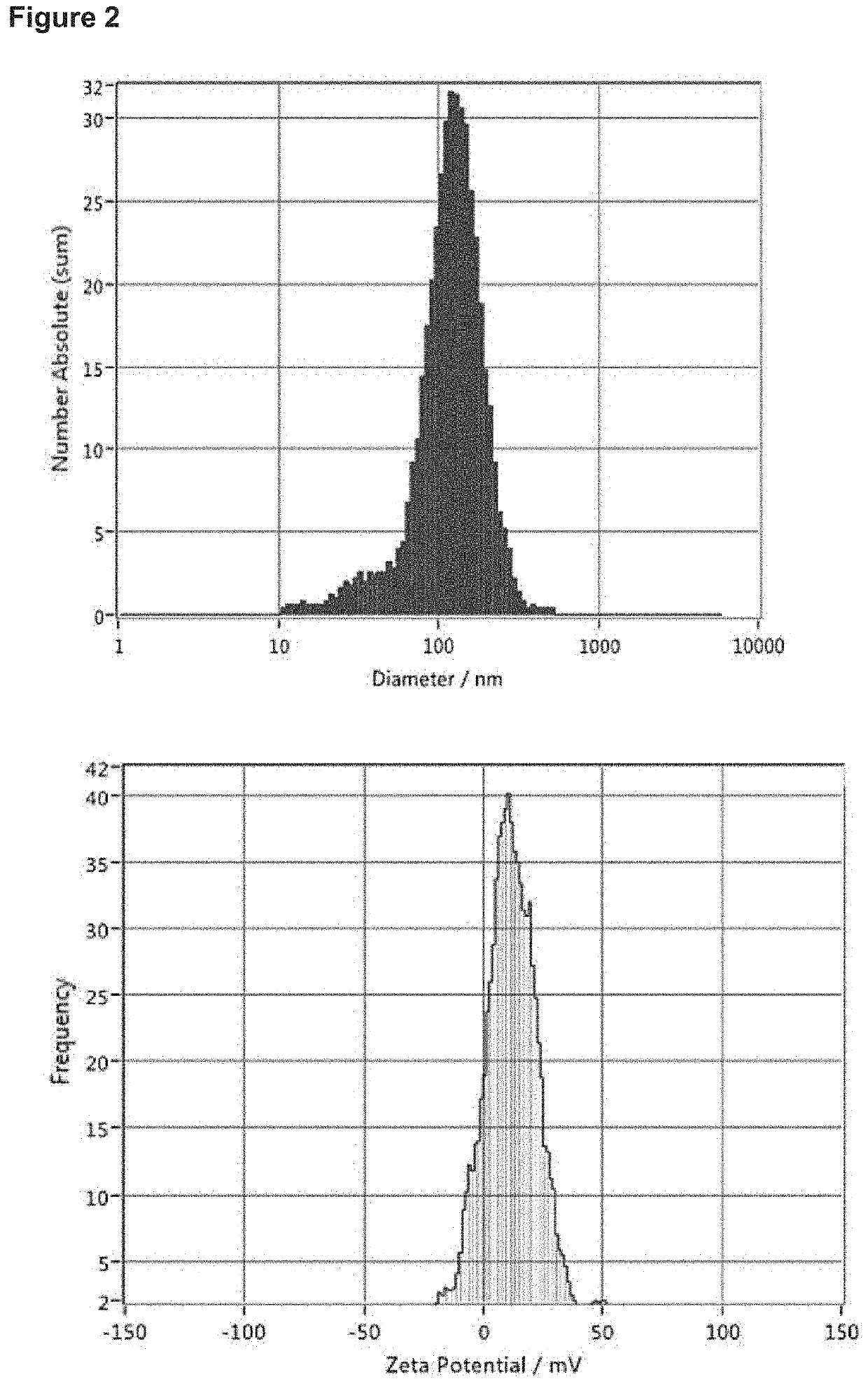
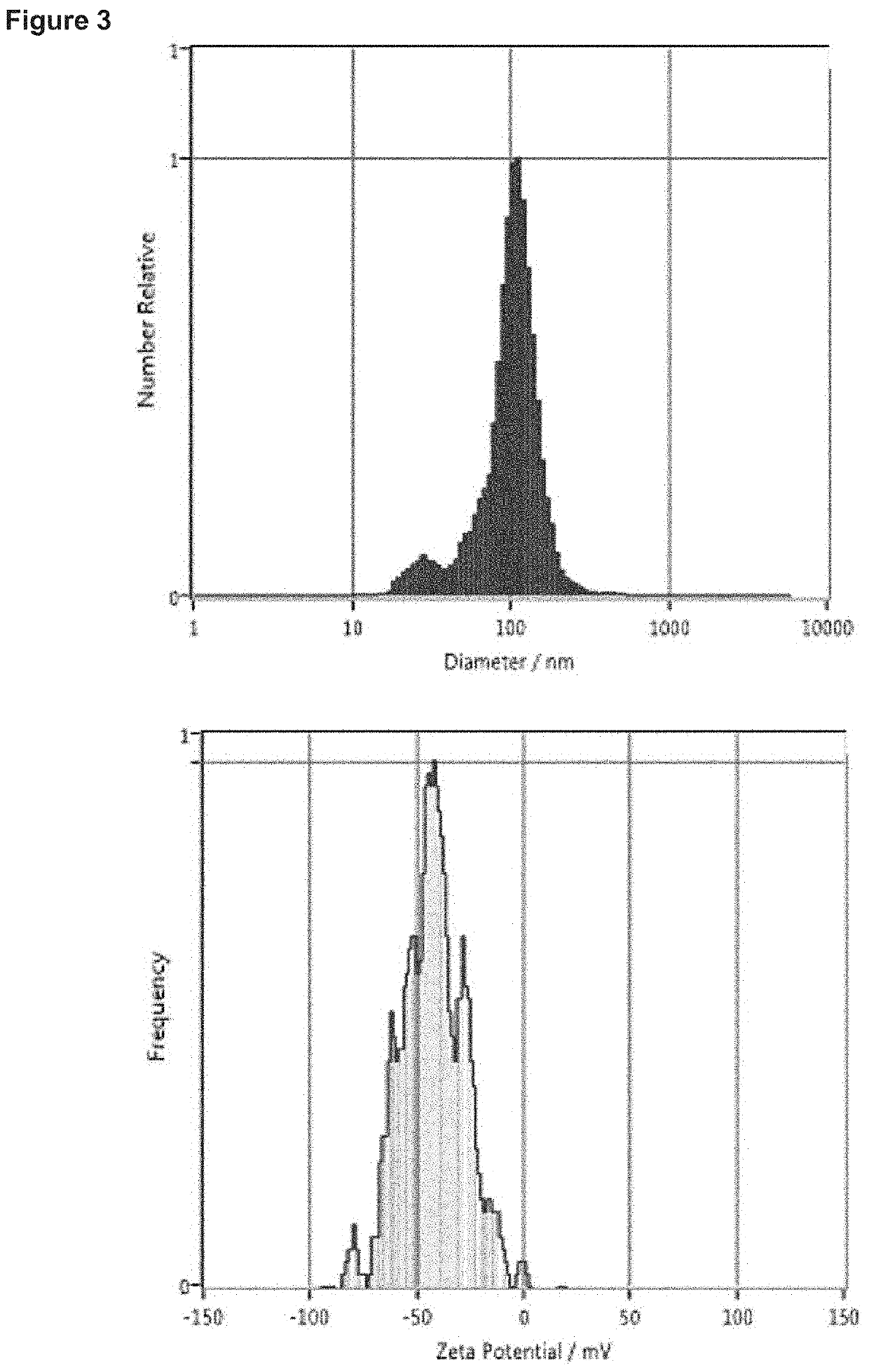
Nanoparticle Core
[0379]A first solution (an anionic solution) was prepared by combining the appropriate amount of payload (in this case plasmid DNA (EGFP-N1 plasmid) with an aqueous mixture (an ‘anionic polymer composition’) of poly(D-glutamic Acid) and poly(L-glutamic acid). This solution was diluted to the proper volume with 10 mM Tris-HCl at pH 8.5. A second solution (a cationic solution), which was a combination of a ‘cationic polymer composition’ and a ‘cationic polypeptide composition’, was prepared by diluting a concentrated solution containing the appropriate amount of condensing agents to the proper volume with 60 mM HEPES at pH 5.5. In this case, the ‘cationic polymer com...
example 3
cle Uptake
[0386]In these studies (e.g., see FIG. 5), nanoparticles with various surface chemistries and charge ratios were tested. Formulations were (charge ratio refers to the nanoparticle core):
[0387]HTT018B: charge ratio (cations / anions) was 2; surface coat was poly(L-Arginine)
[0388]HTT019B: charge ratio was 5; surface coat was poly(L-Arginine)
[0389]HTT020B: charge ratio was 2, surface coat was N-acetyl Semax
[0390]HTT021B: charge ratio was 5, surface coat was N-acetyl Semax
[0391]HTT022B: charge ratio was 2, surface coat was N-acetyl Selank
[0392]HTT023B: charge ratio was 5, surface coat was N-acetyl Selank
[0393]L3000GFP: lipofectamine (non-nanoparticle) delivery of a nucleic acid encoding GFP (plasmid encoding GFP)
[0394]L3000CRISPR: lipofectamine (non-nanoparticle) delivery of CRISPR / Cas components with no GFP or fluorescent tag.
[0395]Nanoparticles were generated. For HTT018B-023B, the core components included a nucleic acid payload (CRISPR / Cas encoding nucleic acids: one plasmid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| branched structure | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com