Method
a technology applied in the field of haematopoietic stem cells and haematopoietic progenitor cells, can solve the problems of affecting the function of hsc, limiting allogeneic hct, and difficulties in using the methods employed for hscs genetic modification, etc., to increase gene transfer efficiency, increase transgen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Highly Purified HSCs are More Transducible by Lentiviral Vectors
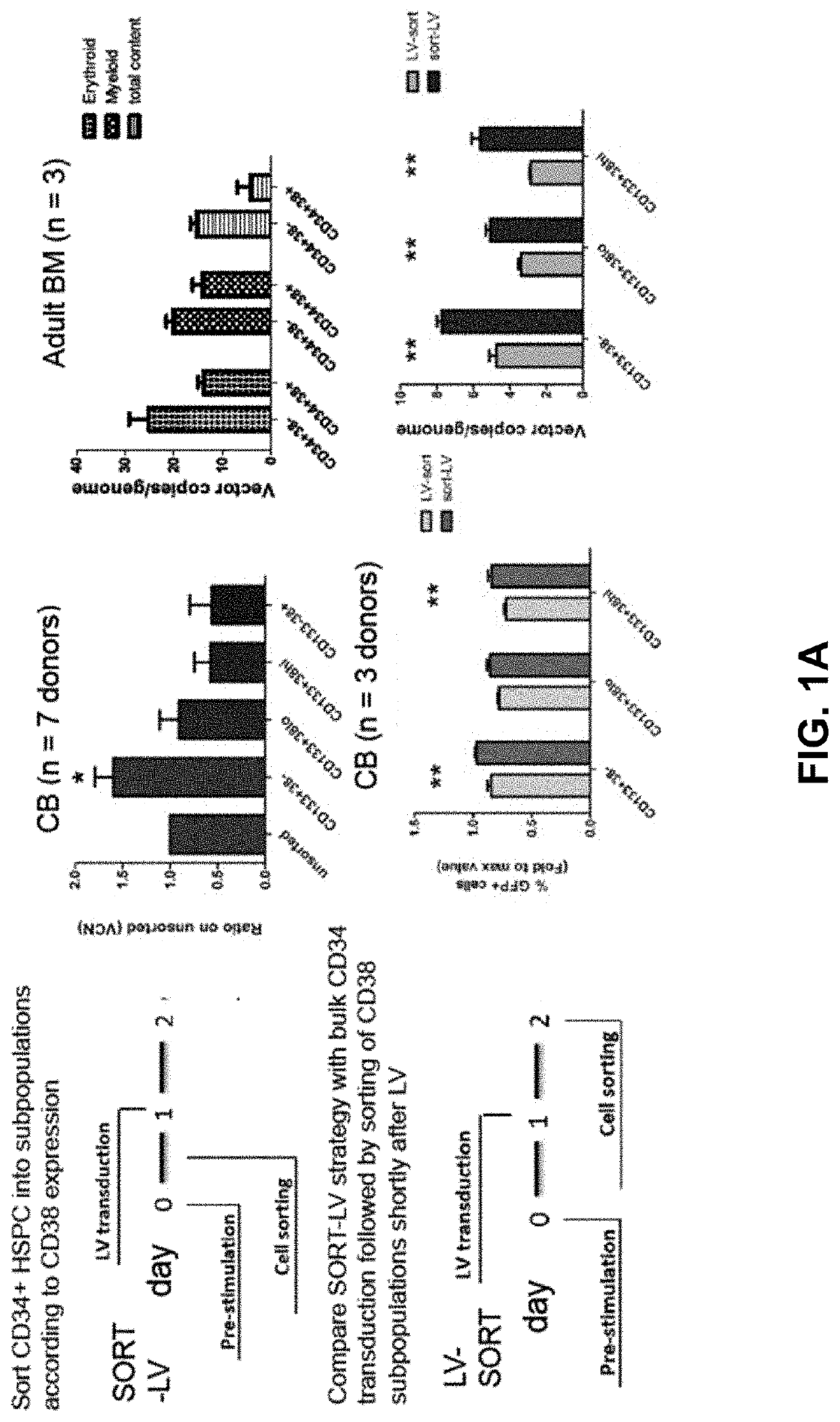
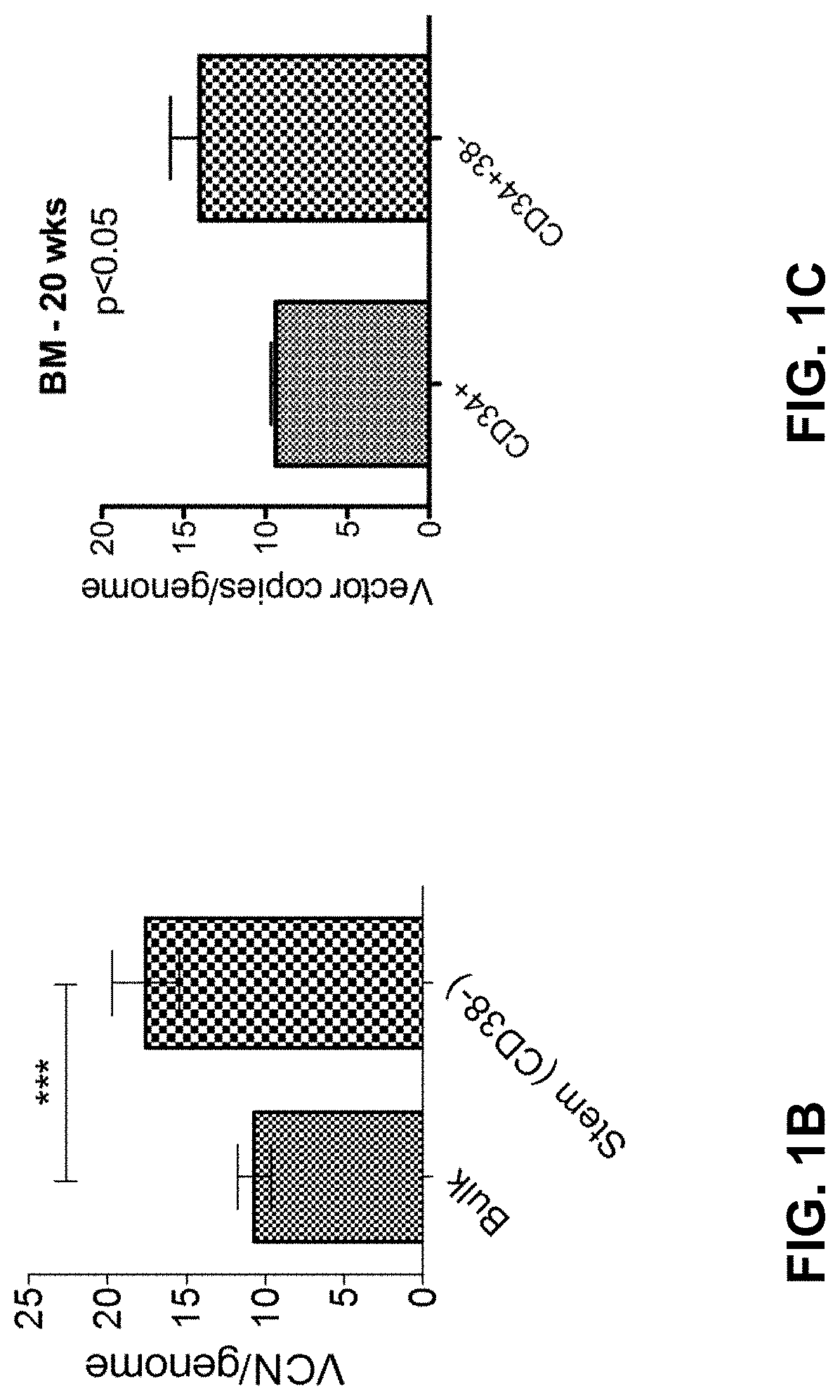
[0312]We studied the differential effect of a microRNA on haematopoietic stem and progenitor cell populations. To this end, CD34+CD38− and CD34+CD38+ cord blood HSPCs were transduced with lentiviral miRNA sponge or overexpressing vectors (data not shown). Unexpectedly, and in contrast to what is widely assumed in the field, we noted a 1.5-fold increased gene transfer into the more primitive, CD34+CD38− HSC-enriched subset. We independently confirmed this observation on multiple cord blood and adult bone marrow donors using biologically neutral vectors expressing marker genes, demonstrating a 1.5 to 2-fold increased gene transfer efficiency into sorted CD34+CD38− HSC-enriched fractions as compared to bulk CD34+ or CD34+CD38+ cell transduction (FIG. 1A).
[0313]Since bulk CD34+ HSPCs contain a small subset of CD38− cells, we wanted to test whether this increased transducibility of more primitive cells was also maintained wi...
example 2
[0316]A bead-based, sequential negative / positive selection for CD38 and CD34, respectively, allows purification of cells with superior NSG engraftment potential
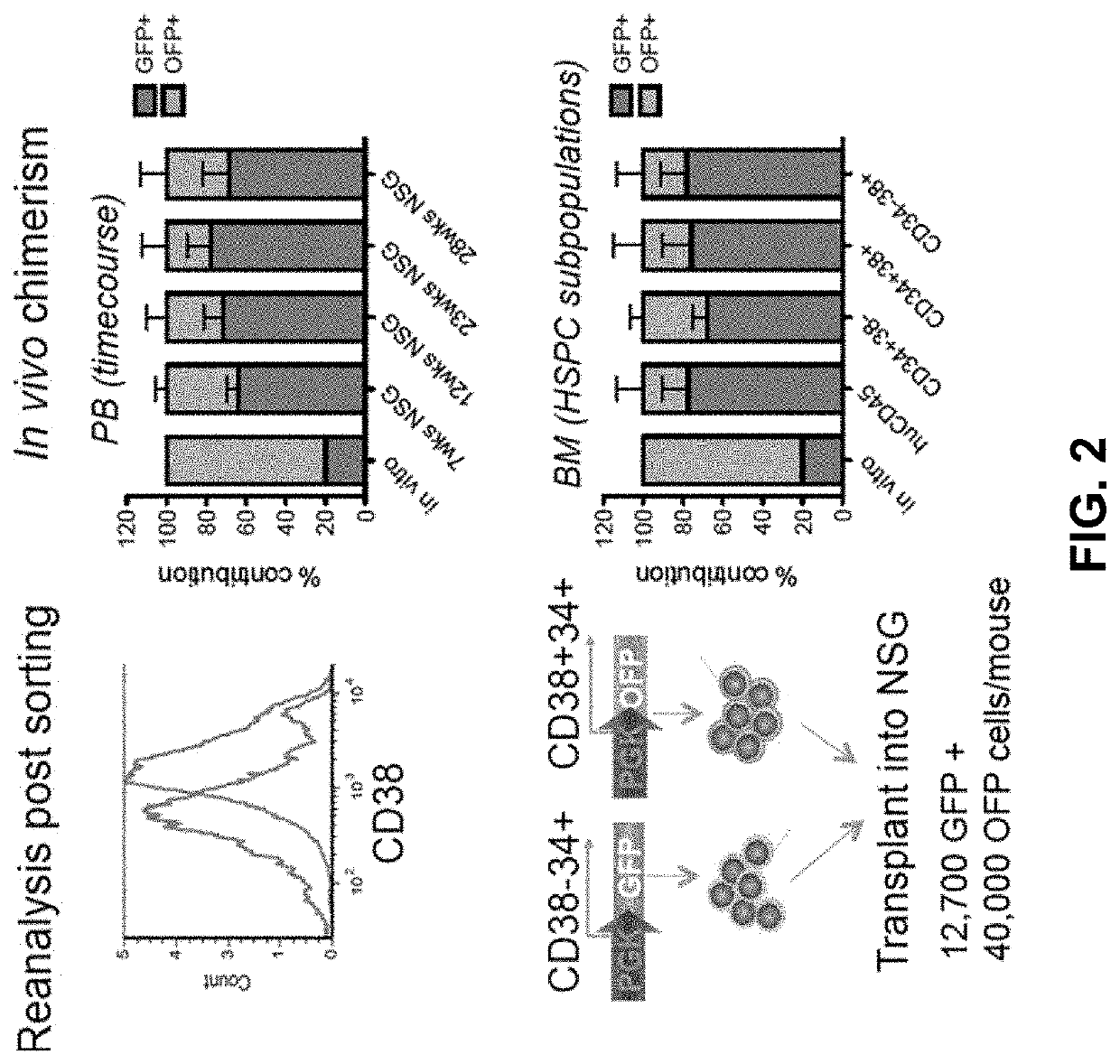
[0317]In order to test the feasibility of a bead-based, sequential negative / positive selection for CD38 and CD34, respectively, we applied a commercially available CD38 selection kit to human cord blood mononuclear cells and tested the engraftment potential of the CD38−(further enriched for CD34 by positive selection) and CD38+ fraction in NSG mice by competitive transplantation (FIG. 2). Even though we used a first generation, non-optimised selection protocol, we could clearly demonstrate an engraftment advantage for the CD38− fraction. While CD38− cells made up less than 20% of the transplant, 70-80% of long-term engraftment was derived from this fraction, motivating further optimisation of this purification protocol.
[0318]Example 3
Modelling a Split Transplant in NSG Mice
[0319]To model the co-transplantation of genetically-...
example 4
[0340]Prostaglandin E2 Increases Gene Transfer into Human Hematopoietic Stem and Progenitor Cells with NSG Repopulating Potential
[0341]In an effort to exploit the anti-apoptotic properties of prostaglandin E2 (PGE2) (Pelus LM et al. Prostaglandins Other Lipid Mediat. 2011;96:3-9), we treated CD34+ HSPC exposed to stress (freeze / thaw cycles, transduction with toxic vectors, electroporation) with the long acting PGE2 homologue 16,16-dimethyl Prostaglandin E2 (dmPGE2; Cayman Chemical, Cat 14750). Unexpectedly, we found a 1.5 fold increased gene transfer efficiency into CD34+ or CD34+CD38− HSPC from cord blood (FIG. 5A) and adult bone marrow (FIG. 6) after dmPGE2 treatment. This difference in vector copy number (VCN) was maintained over more than 4 months after transplantation of the cells into NSG mice, and engraftment levels were not negatively affected by dmPGE2 treatment.
[0342]We have confirmed that G-CSF-mobilised CD34+ peripheral blood stem cells also undergo more efficient transd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com