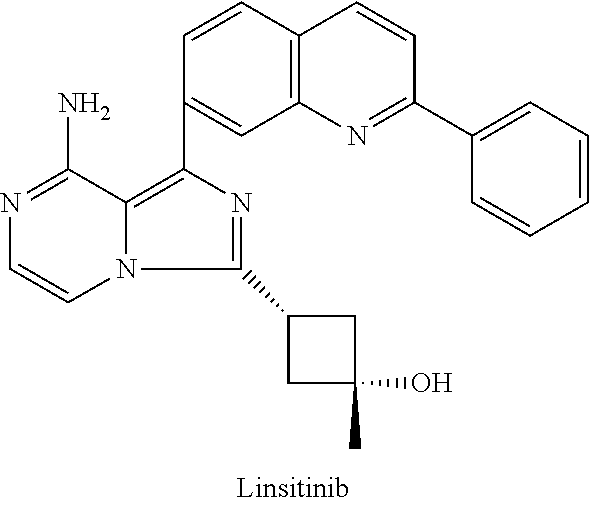
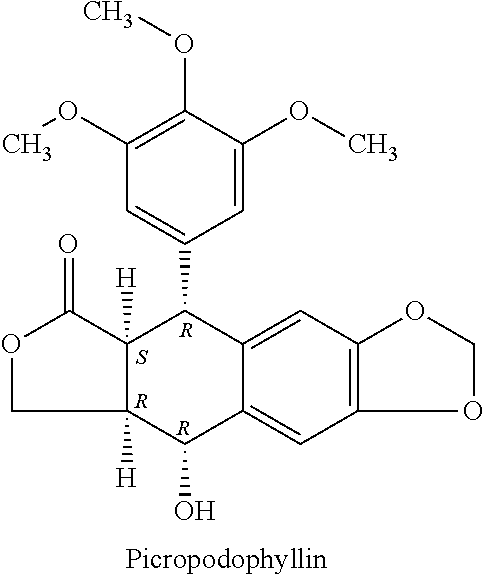
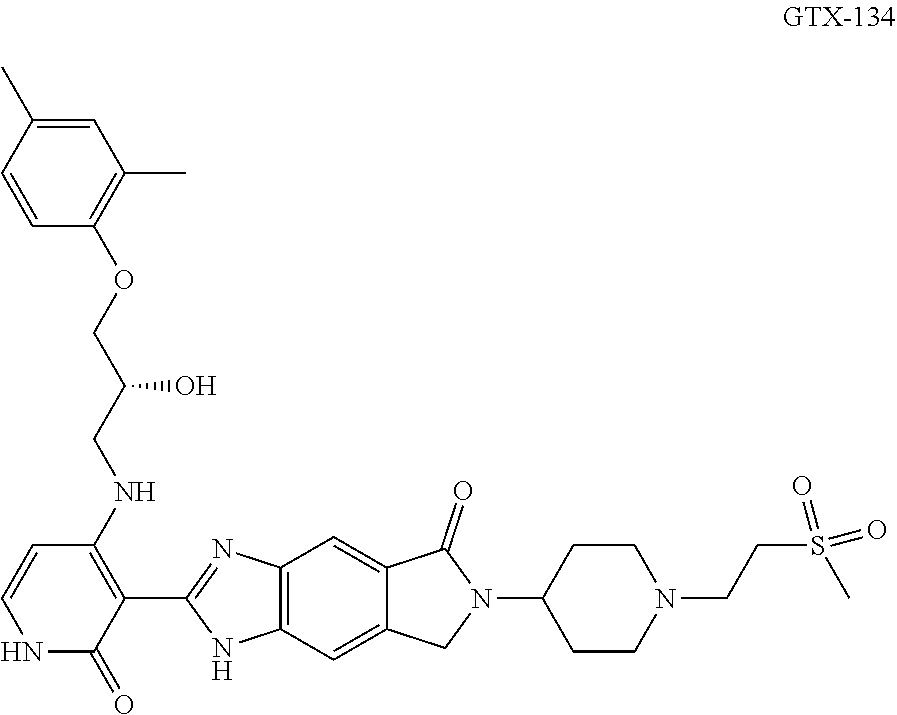
Methods for the treatment of thyroid eye disease
a thyroid eye disease and eye disease technology, applied in the field of thyroid eye disease treatment, can solve the problems of insufficient treatment of ted (tao or go), limited efficacy of medical therapies, and safety concerns, so as to reduce the severity of ted eye disease, improve the quality of life of ted patients, and reduce the effect of proptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0021]Embodiment 1. A method of treating thyroid eye disease (TED), comprising administering to the subject an effective amount of an insulin like growth factor-I receptor (IGF-1R) inhibitor.
[0022]Embodiment 2. A method of reducing proptosis by at least 2 mm in a subject with thyroid eye disease (TED), comprising administering to the subject an effective amount of an IGF-IR inhibitor.
[0023]Embodiment 3. The method of Embodiment 2, wherein proptosis is reduced by at least 3 mm.
[0024]Embodiment 4. The method of Embodiment 3, wherein proptosis is reduced by at least 4 mm.
[0025]Embodiment 5. The method of Embodiment 2, wherein the method additionally comprises reducing the clinical activity score (CAS) in the subject with TED.
[0026]Embodiment 6. The method of Embodiment 5, wherein CAS is reduced by at least 2 points.
[0027]Embodiment 7. The method of Embodiment 6, wherein CAS is reduced by at least 3 points.
[0028]Embodiment 8. The method of Embodiment 7, wherein proptosis is reduced by a...
examples
[0413]Exemplary embodiments are provided in the following Examples 1-X. The following examples are presented only by way of illustration and to assist one of ordinary skill in using the invention. The examples are not intended in any way to otherwise limit the scope of the invention. In some embodiments, said IGF-1R inhibitor is an antibody or a subset of antibodies chosen from amongst the Examples below. In some embodiments, said IGF-1R inhibitor is a small molecule or a subset of small molecules chosen from amongst the Examples below.
example a
Teprotumumab
[0414]Provided first is teprotumumab (TEPEZZA), an IGF-1R inhibitor approved for the treatment of TED. Teprotumumab and other related IGF-1R inhibitor antibodies and their methods of preparation can be found in U.S. Pat. No. 7,572,897, US20190225696, and US20190270820, which are hereby incorporated by reference in their entireties. In certain embodiments, teprotumumab may be used as an active control in clinical trials of other IGF-1R inhibitors, e.g. as in Example 31.
TABLE ATeprotumumab Sequences and SEQ ID NumbersSEQIDNODescriptionSequenceAntibody 1(teprotumumab)1CDRH1 aaSer Tyr Gly Met His2CDRH2 aaIle Ile Trp Phe Asp Gly Ser Ser Thr Tyr Tyr Ala Asp Ser Val ArgGly3CDRH3 aaGlu Leu Gly Arg Arg Tyr Phe Asp Leu4CDRL1 aaArg Ala Ser Gln Ser Val Ser Ser Tyr Leu Ala5CDRL2 aaAsp Ala Ser Lys Arg Ala Thr6CDRL3 aaGln Gln Arg Ser Lys Trp Pro Pro Trp Thr7VH aaGln Val Glu Leu Val Glu Ser Gly Gly Gly Val Val Gln Pro GlyArg Ser Gln Arg Leu Ser Cys Ala Ala Ser Gly Phe Thr Phe SerSer Tyr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| average distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com