Methods of treating cancer
a cancer and cancer technology, applied in the field of cancer treatment methods, can solve the problems of poor prognosis of many cancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Phase 1 Study of Drug A in Combination With Established Anticancer Antibodies in Patients With Advanced Malignancies
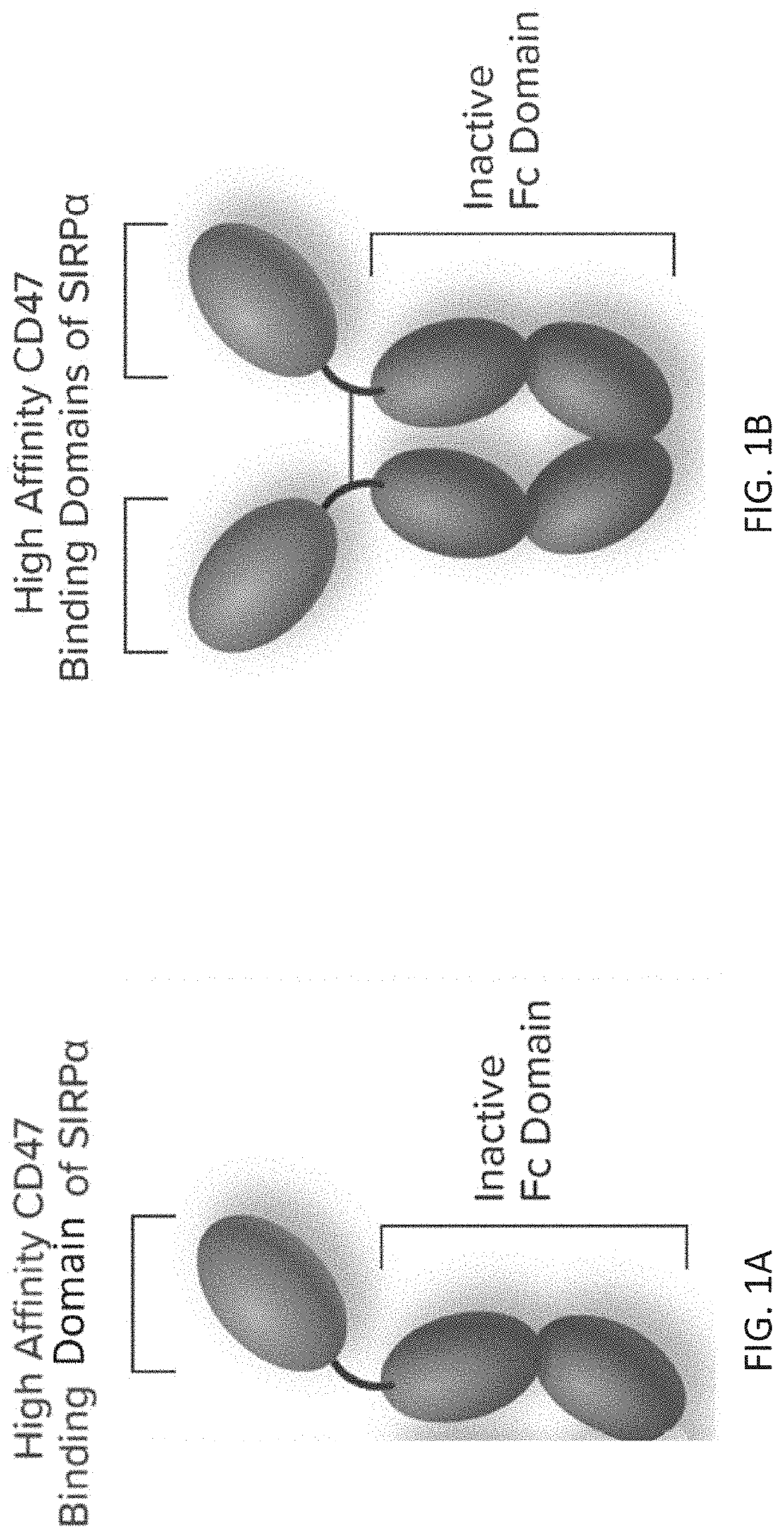
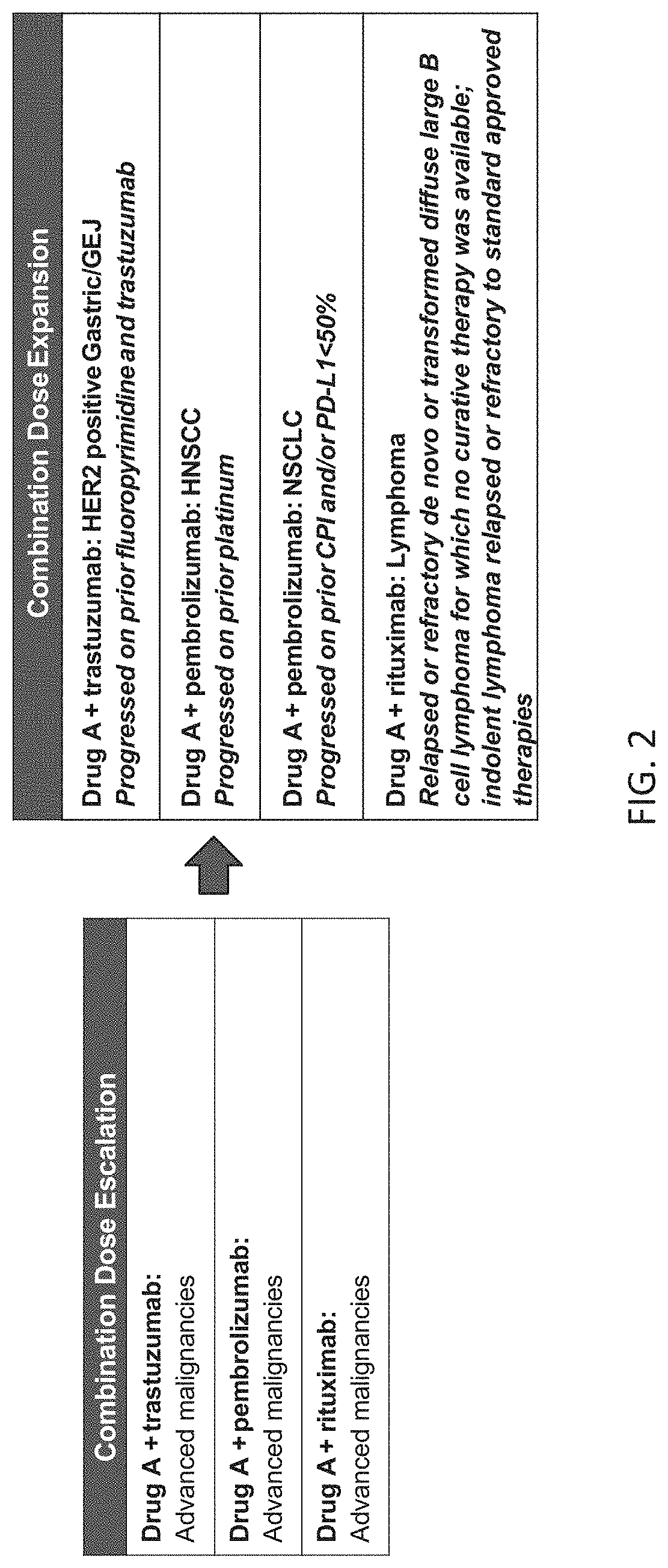
[0329]This Example describes a Phase 1 clinical study that evaluated the safety, efficacy, pharmacodynamics (PD) and pharmacokinetics (PK) of Drug A in combination with pembrolizumab, trastuzumab, or rituximab for patients with advanced malignancies. Drug A is a fusion protein consisting of a high affinity CD47-binding SIRPα D1 domain variant fused to a human immunoglobulin Fc domain variant that is modified to eliminate binding to Fc gamma receptors (FIG. 1).
[0330]Study Objectives
[0331]Primary Objective
[0332]The primary objective of this study was to evaluate the safety and tolerability of Drug A administered once every week and / or every 2 weeks in combination with pembrolizumab, trastuzumab, or rituximab in patients with advanced malignancies including non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), HER2-overexpressing gastric can...
example 2a
Further Safety Results From a Phase 1 Study of Drug A in Combination With Pembrolizumab or Trastuzumab
[0459]As described in Example 1, 52 patients with solid tumor received Drug A in combination with pembrolizumab, and 30 patients with solid tumor received Drug A in combination with trastuzumab. Treatment-related adverse effects (TRAEs) (including fatigue, AST increase, platelet decrease, ALT increase, anemia, and / or pruritus) were of low grade and low frequency.
[0460]35 (67.3%) of patients who received Drug A+ pembrolizumab and 22 (73.3%) of patients who received Drug A+ trastuzumab experienced any TRAE. The most frequent TRAE experienced by patients who received Drug A+ pembrolizumab was low-grade aspartate transaminase (AST) increase (17.3%), and the most frequent TRAE experienced by patients who received Drug A+ trastuzumab was low grade fatigue (30%). TRAEs of ≥Grade 3 severity were of low frequency. See Tables E and F below.
TABLE ETRAE in Patients Receiving Drug A + Trastuzuma...
example 2b
Further Results From a Phase 1 Study of Drug A in Combination With Pembrolizumab in Patients With ≥2L Head and Neck Squamous Cell Carcinoma
[0463]As described in Example 1, 20 patients with ≥2 L HNSCC received Drug A in combination with pembrolizumab. Baseline characteristics of all patients receiving Drug A+ pembrolizumab (including patients with ≥2L NSCLC, as described in further detail in Example 2C below) are shown in Table G.
TABLE GBaseline Characteristic of All Patients* Receiving Drug A + PembrolizumabDrug A + PembrolizumabN = 52Primary Disease, nLung25HNSCC20Gastric / GEJ—EsophagealBreast—Colorectal2Ovarian2Pancreatic—Appendiceal1Sarcoma1Urothelial—Unknown1Median AgeYears (range)61 (32-81)Sex, nM29F23Race, nWhite34Asian11Black3Other4ECOG PS, n018134*patients included those with ≥ 2 L HNSCC and ≥ 2 L NSCLC (see Example 2C).
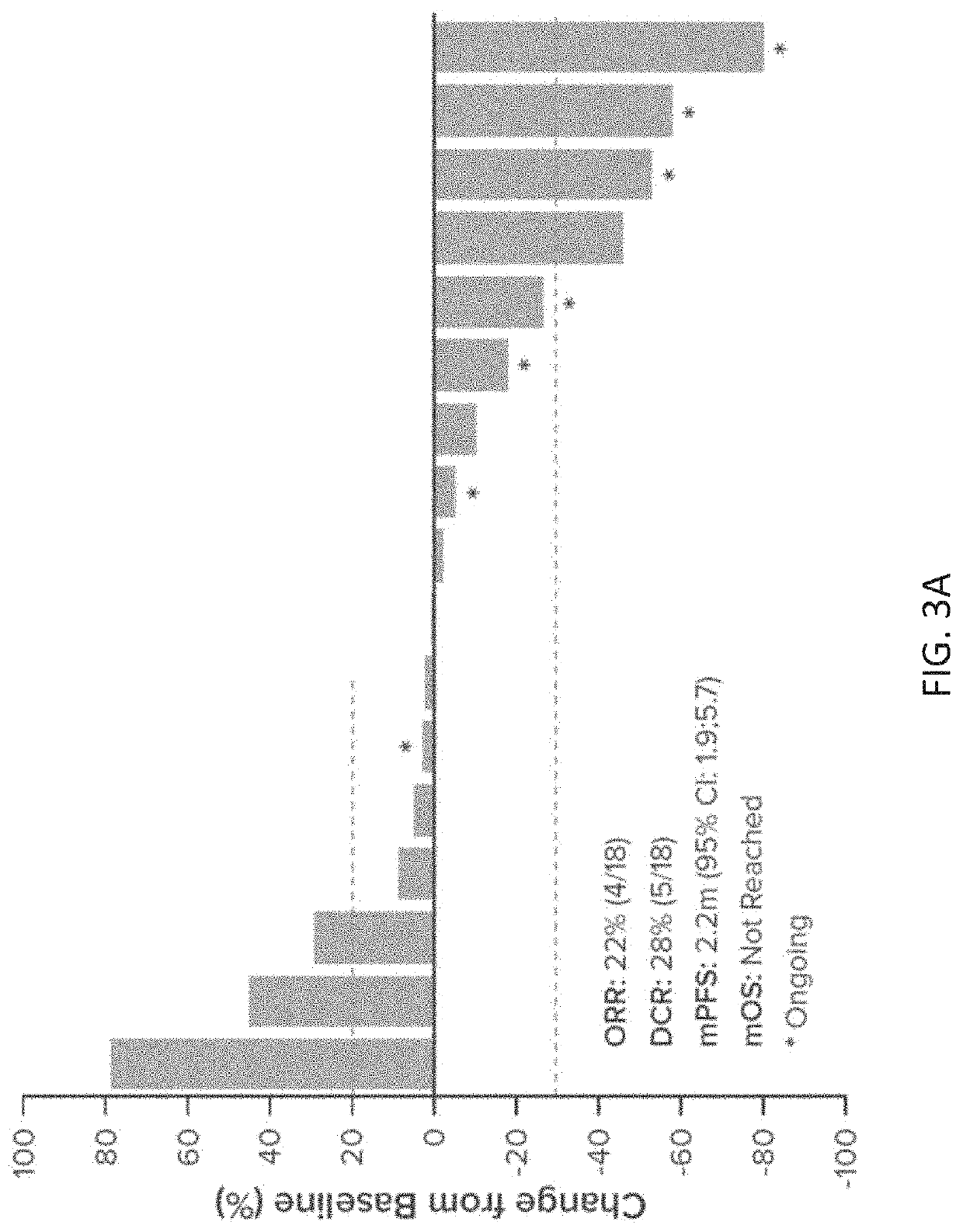
[0464]Anticancer efficacy in HNSCC patients was observed in response-evaluable patients. Clinical activity was based on investigator assessed response using R...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com