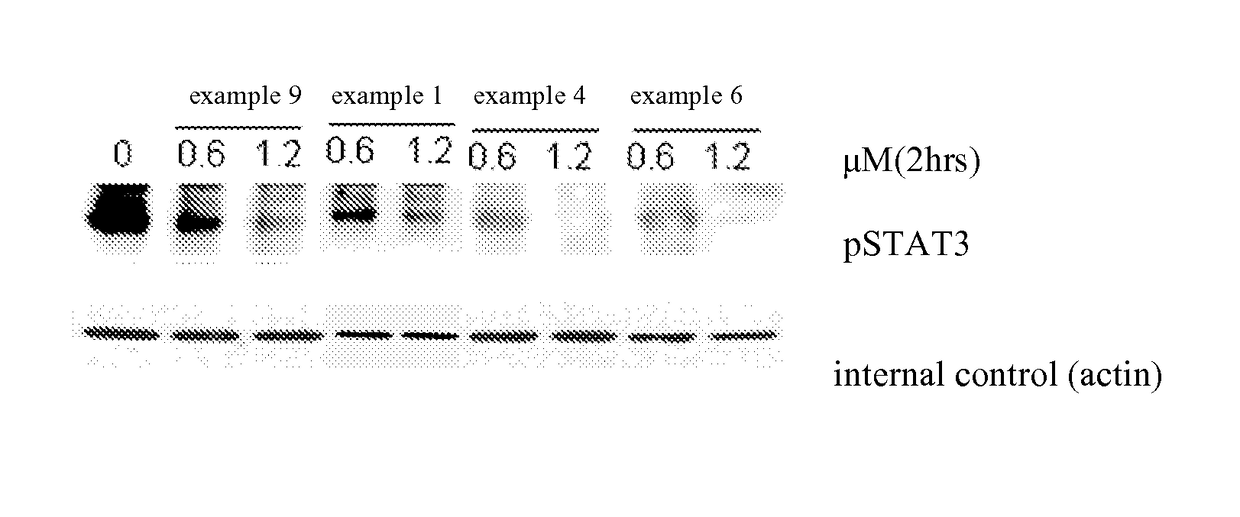
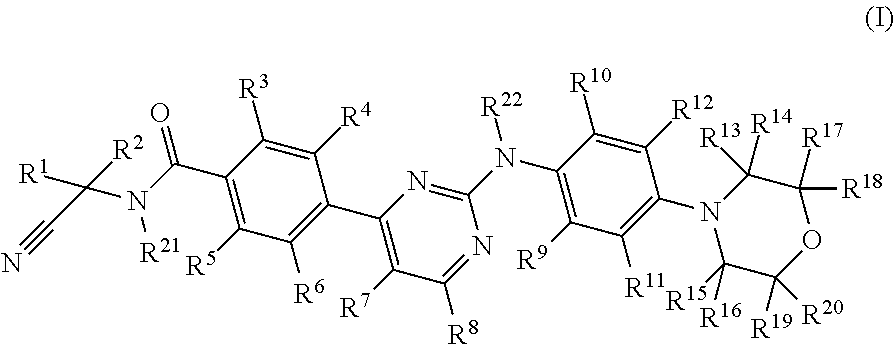
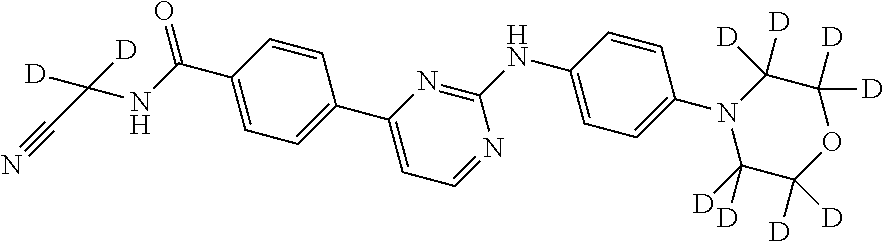
Deuterated phenyl amino pyrimidine compound and pharmaceutical composition containing the same
a phenylaminopyrimidine and compound technology, applied in the field of new deuterated phenylaminopyrimidine compounds, can solve the problems of drug resistance problems, side effects, druggability problems, etc., and achieve the effect of improving pharmacodynamic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
the preparation of N-(cyanomethyl)-4-(2-(4-(d8-morpholino)phenyl amino)pyrimidine-4-yl)benzamide (compound 9)
[0107]
1. The preparation of 4-(4-nitrophenyl) (d8-morpholine) (compound 3)
[0108]Compound 4-chloronitrobenzene (3.53 g, 22.4 mmol), d8-morpholine (2.35 g, 24.6 mmol) and potassium carbonate (6.07 g, 44 mmol) were added into a flask sequentially, and dimethylsulfoxide (40 mL) was added. After that, it was heated up to 100° C. and was stirred for 16 h. TLC analysis (ethyl acetate / petroleum ether=1 / 10) showed that the reaction has completed. Then it was cooled to room temperature, and the reaction was quenched by adding water (100 mL). It was extracted by dichloromethane (100 mL) for two times. The combined organic layer was washed with water and saturated brine, dried with anhydrous sodium sulfate, filtered and concentrated under vacuum by rotary evaporator to give the crude product. It was crystallized in the mixed solvent of ethyl acetate and n-hexane (1 / 5, v / v, 18 mL) to obta...
example 2
The preparation of N-(cyanomethyl)-4-(2-(4-(d8-morpholino)phenyl amino)pyrimidine-4-yl)benzamide (compound 9)
[0113]
1. The preparation of 4-(4-nitrophenyl) (d8-morpholine) (compound 3)
[0114]4-chloronitrobenzene (0.662 g, 4.17 mmol), d8-morpholine (0.400 g, 4.17 mmol, purchased from Cambridge Isotope Laboratories) and potassium carbonate (1.733 g, 12.54 mmol) were added to a flask sequencially. Dimethylsulfoxide (6 mL) was added, then heated to 100° C., and stirred for 20 hours. The reaction mixture was cooled to room temperature, then water (30 mL) was added to quench the reaction, and there was yellow solid precipitated. After stirred for 30 min, the mixture was filtered to obtain the crude product. The crude product was crystallized in the mixed solvent of ethyl acetate and petroleum ether (1 / 2.5, v / v, 14 mL) to obtain the yellow solid desired product (0.600 g, HPLC purity: 98.6%, yield 67%).
2. The preparation of 4-(d8-morpholino)phenylamine (compound 4)
[0115]4-(4-nitrophenyl) (d8-...
example 3
The preparation of N-(cyano(d2-methyl))-4-(2-(4-(d8-morpholino)phenylamino)pyrimidin-4-yl)benzamide (compound 13)
1. The preparation of 2-amino-2,2-d2-acetonitrile hydrochloride (compound 12)
[0120]
[0121]Sodium cyanide (1.59 g, 32.46 mmol) and ammonia (6.80 g, 99.87 mmol) was added to the flask under ice bath, the inner temperature was controlled under 5° C., and ammonium chloride (2.27 g, 42.25 mmol) was added. After 10 min of stirring, deuterated formaldehyde (1.00 g, 31.21 mmol) was slowly added dropwise in 10 mins, and the inner temperature was controlled to be 16-20° C. After stirred under heat-preservation for 3 h, dichloromethane (100 ml) was added and stirred for 45 min, and liquid separated to obtain organic phase. The aqueous phase was extracted twice with dichloromethane (100 ml×2), the combined organic phase was dried over anhydrous sodium sulfate, and filtered. A solution of hydrogen chloride in isopropanol (15 mL) was added to the filtrate under ice bath, and was stirred...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| reaction time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com