Protective cap
a protective cap and membrane technology, applied in the field of protective caps, can solve the problems of high cost, increased increased risk of medical and pharmacological staff being exposed to drugs or solvents which may escape into ambient air, etc., and achieve the effects of increasing friction and/or mechanical engagement, improving the attachment between the membrane and the membrane holder, and reducing the risk of exposur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
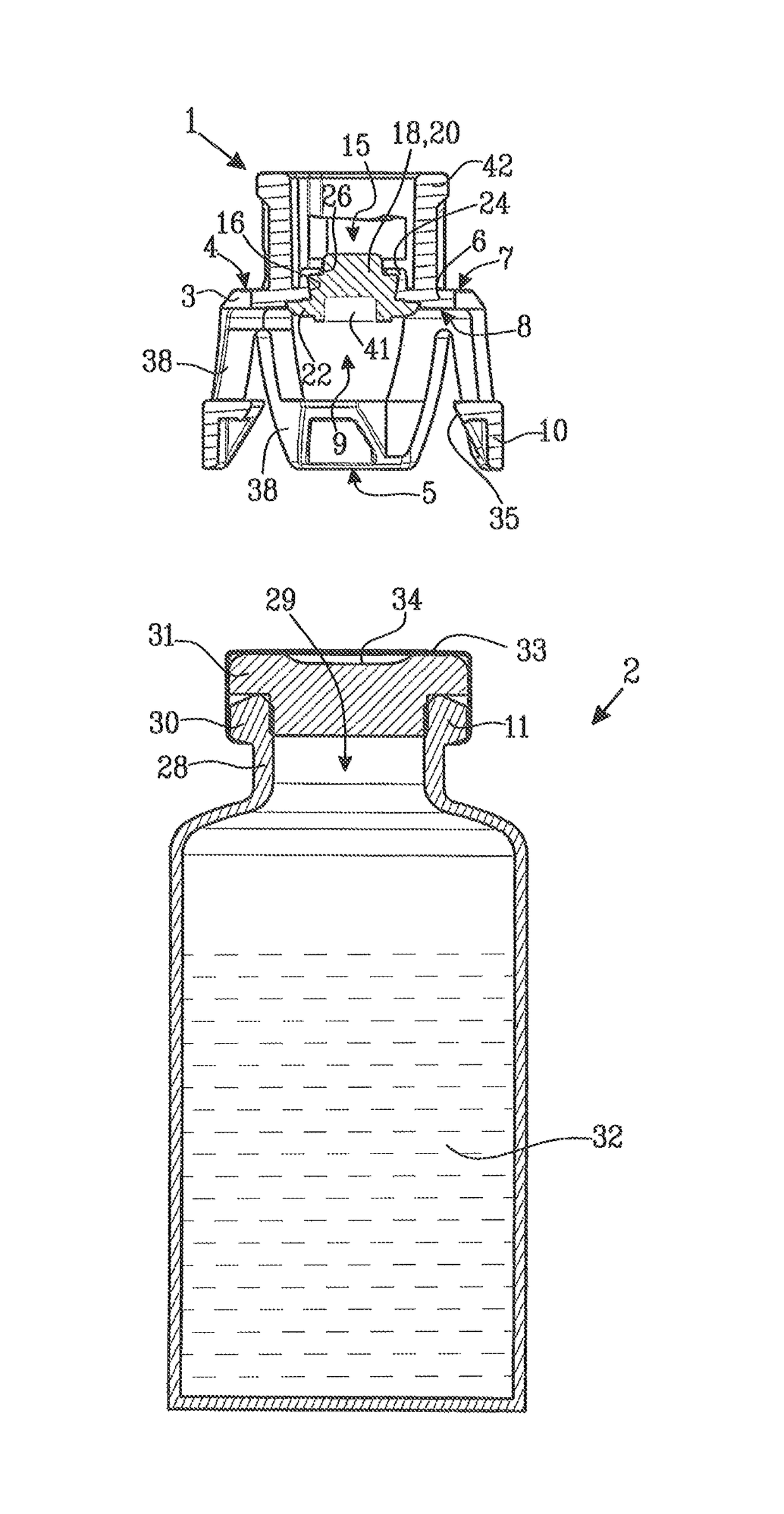
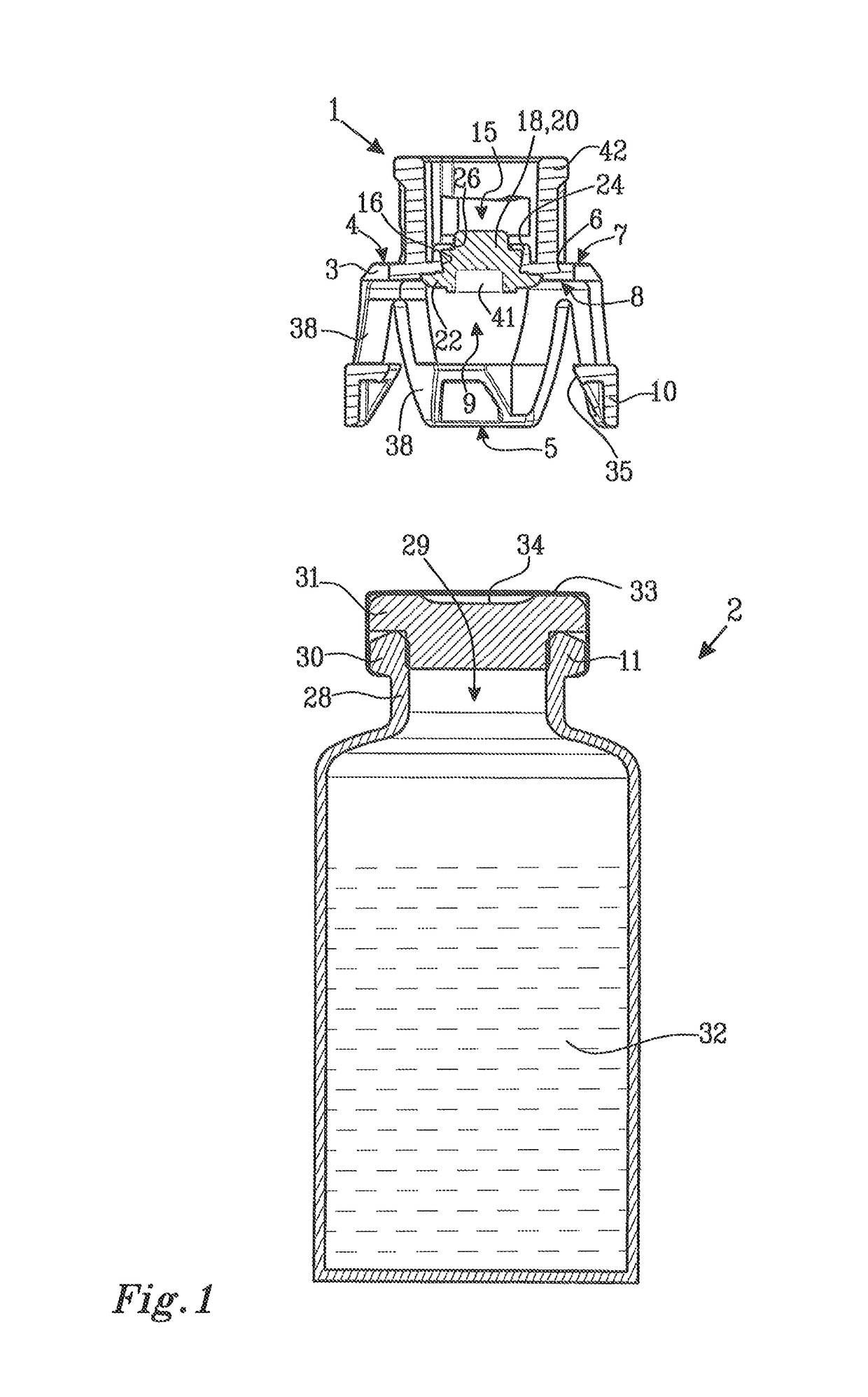
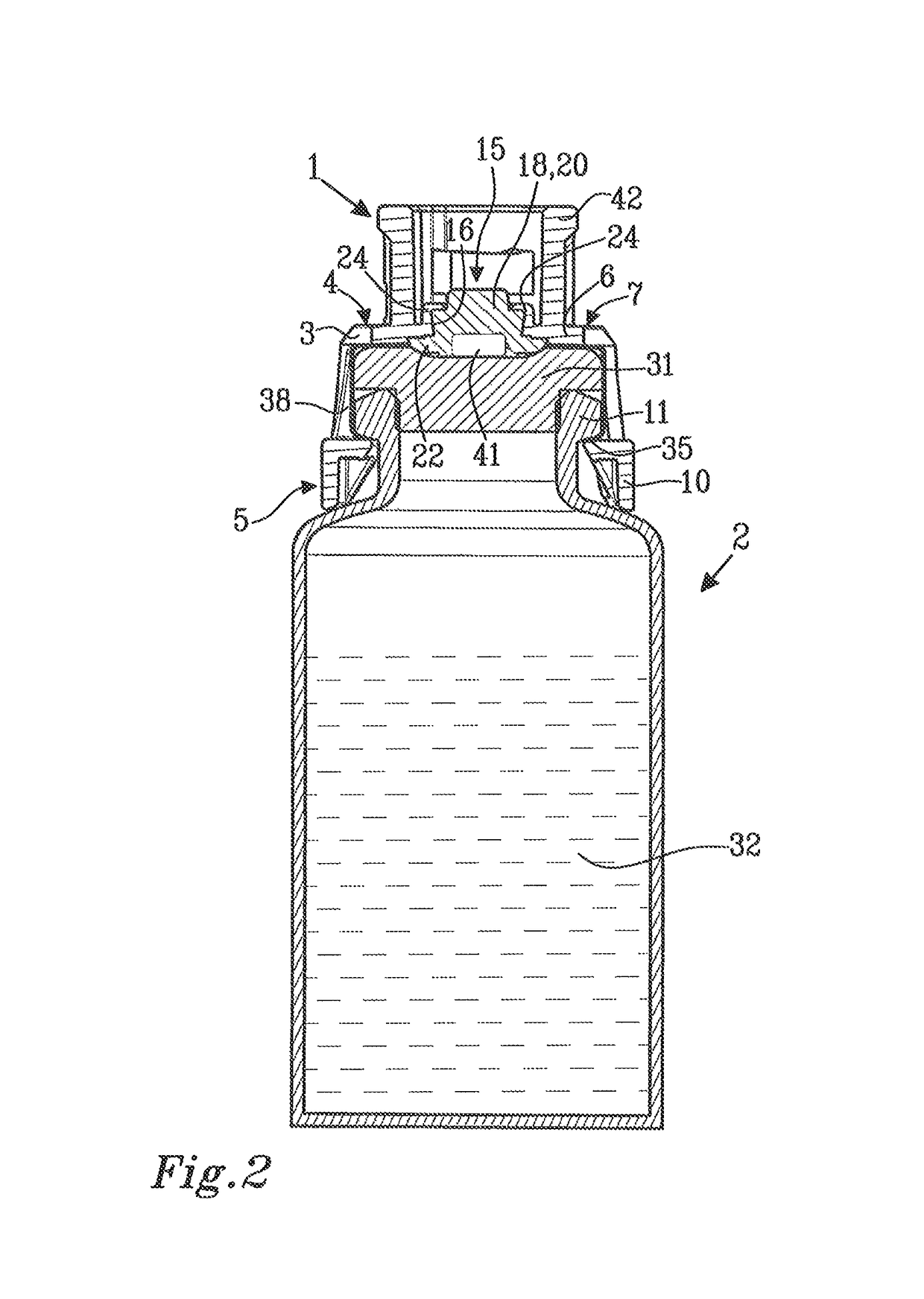
[0035]FIG. 1 shows a protective cap 1 and a medical device in the form of a vial 2. The protective cap 1 comprises a membrane holder 3 having a first end 4 with an end wall 6. The end wall 6 has an outer surface 7 and an inner surface 8. A second end 5 is arranged at a distance from the first end 4 and is provided with an end opening 9. The second end 5 is arranged to be connected to the vial 2 and is provided with first connection means 10 placed at the periphery of the end opening 9 and intended to engage with cooperating second connection means 11 on the vial 2.
[0036]The end wall 6 of the membrane holder 3 is provided with a central piercing opening 15. The piercing opening 15 has a peripheral edge 16. A resilient membrane 18 is arranged to cover the piercing opening 15. The resilient membrane 18 has a piercing portion 20 and a sealing portion 22 peripherally surrounding the piercing portion 20.
[0037]The resilient membrane 18 is attached to the membrane holder 3 by mechanical hol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com