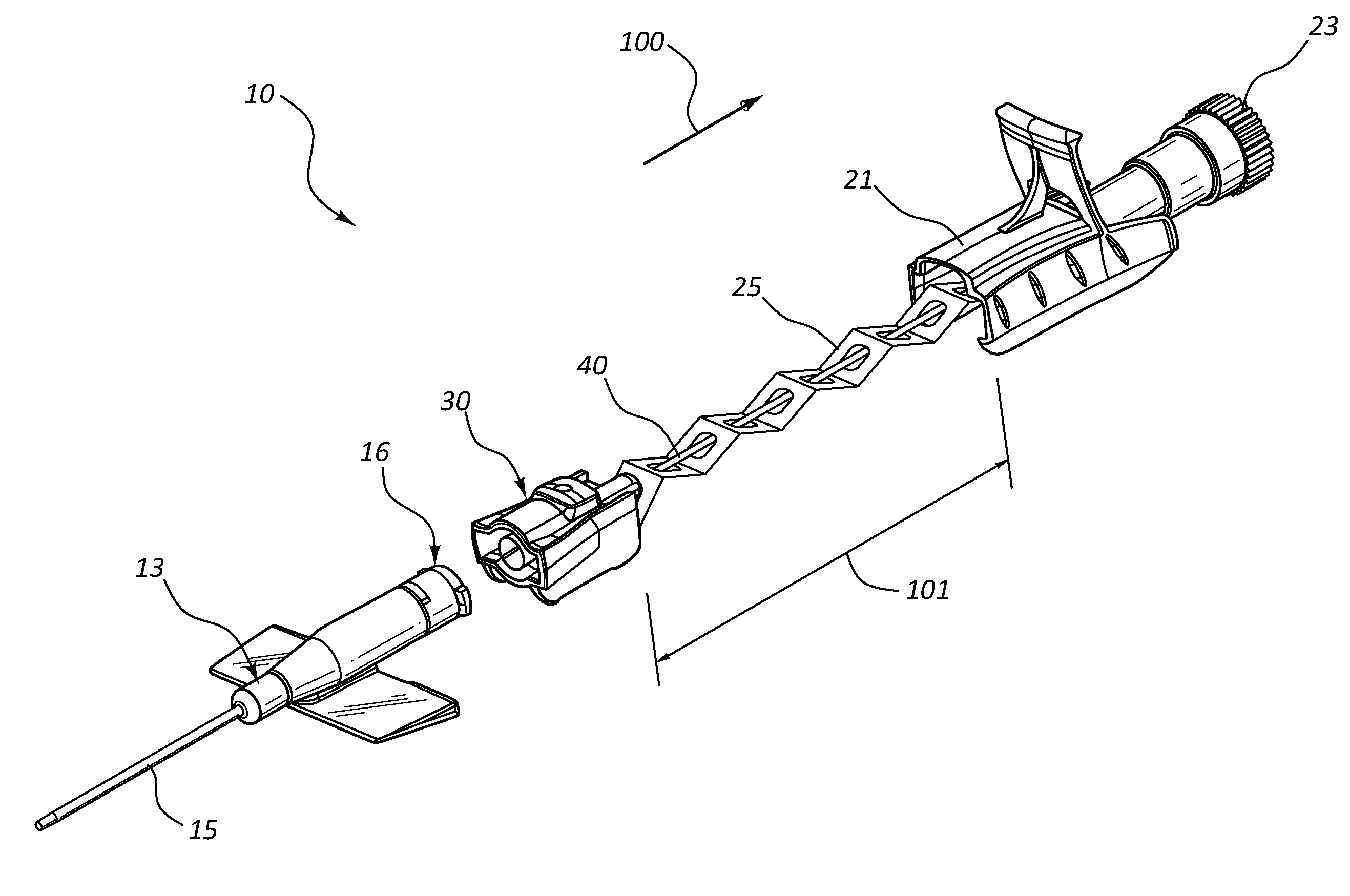
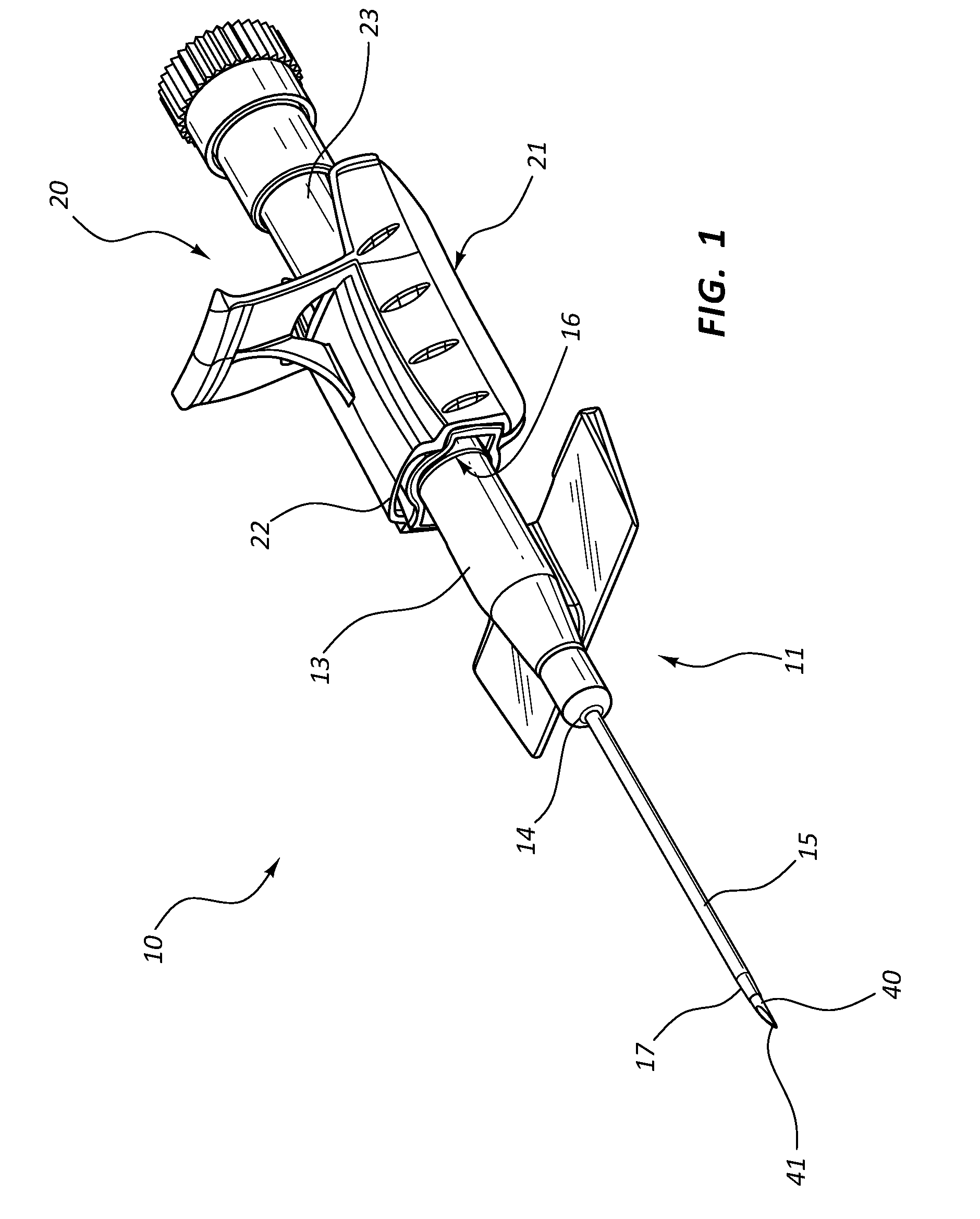
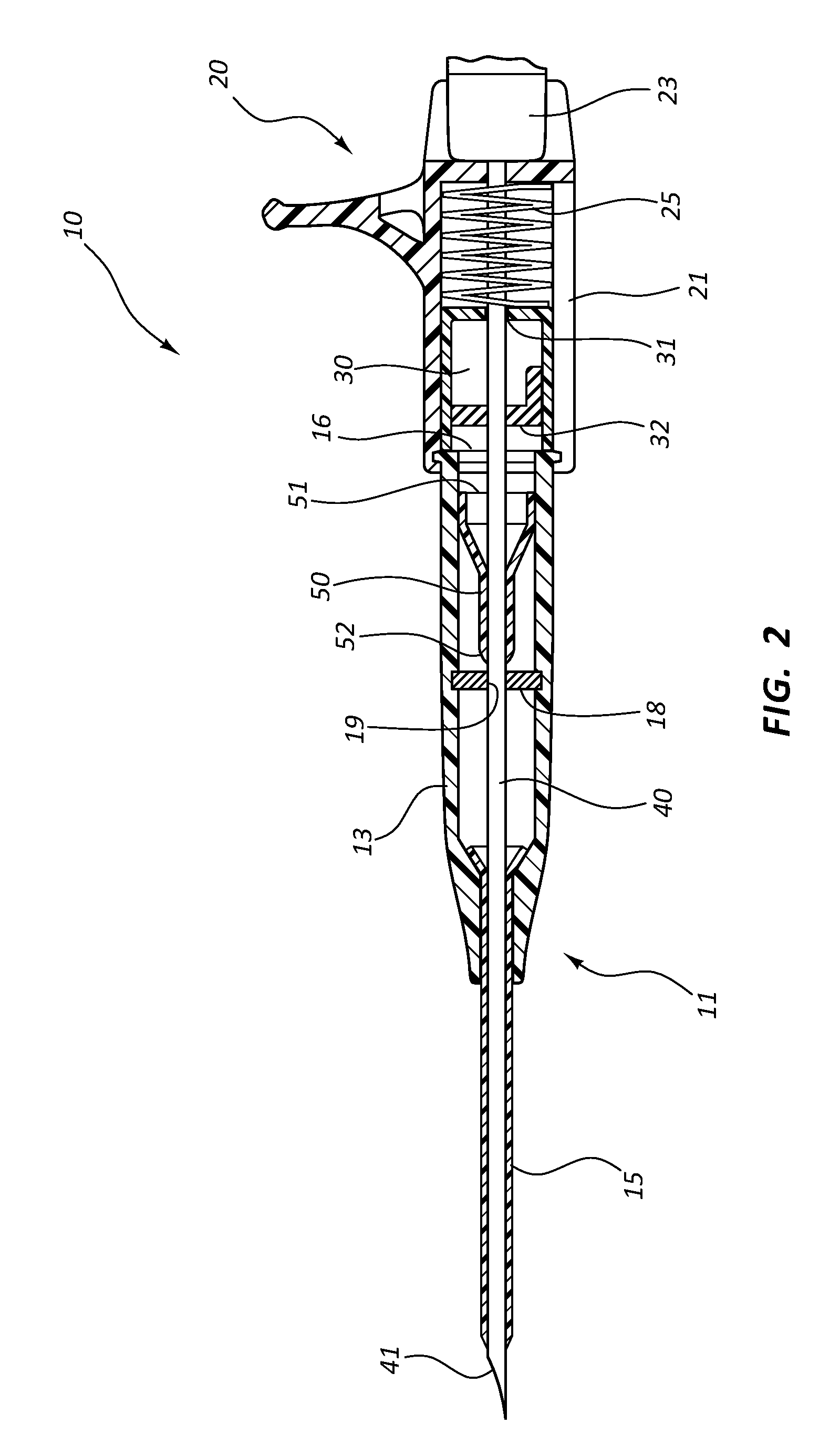
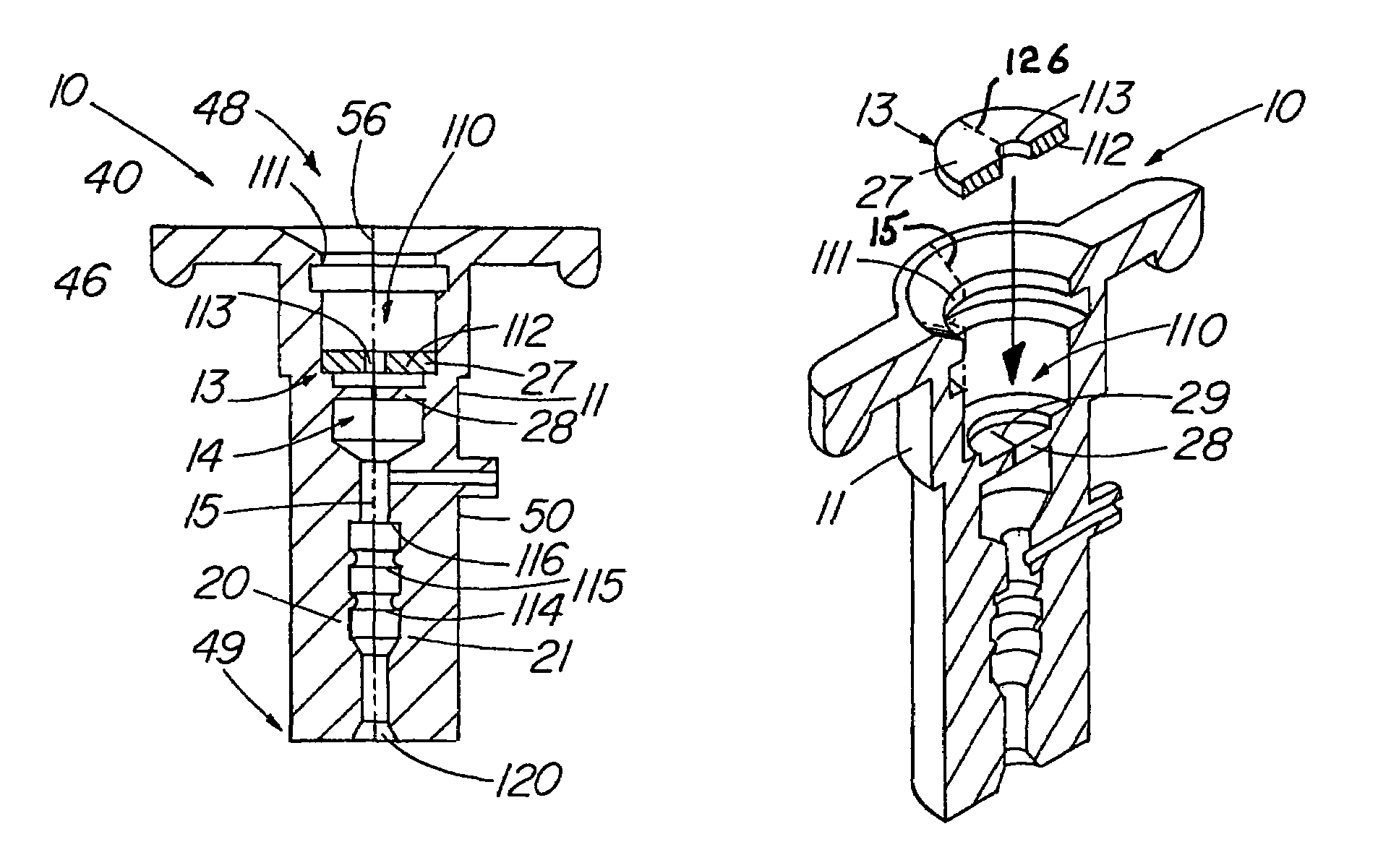
Patents
Literature
1778results about "Other medical devices" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
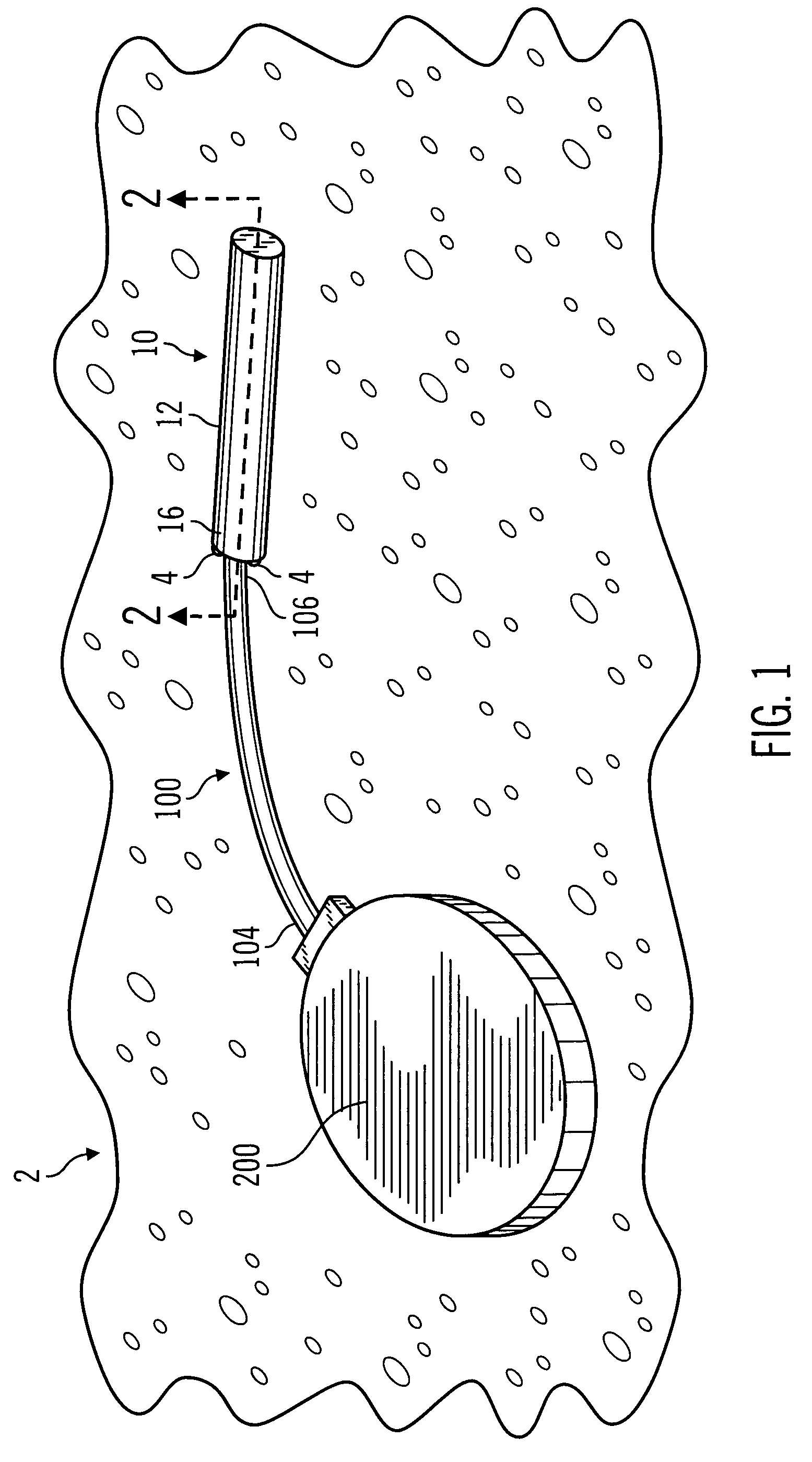
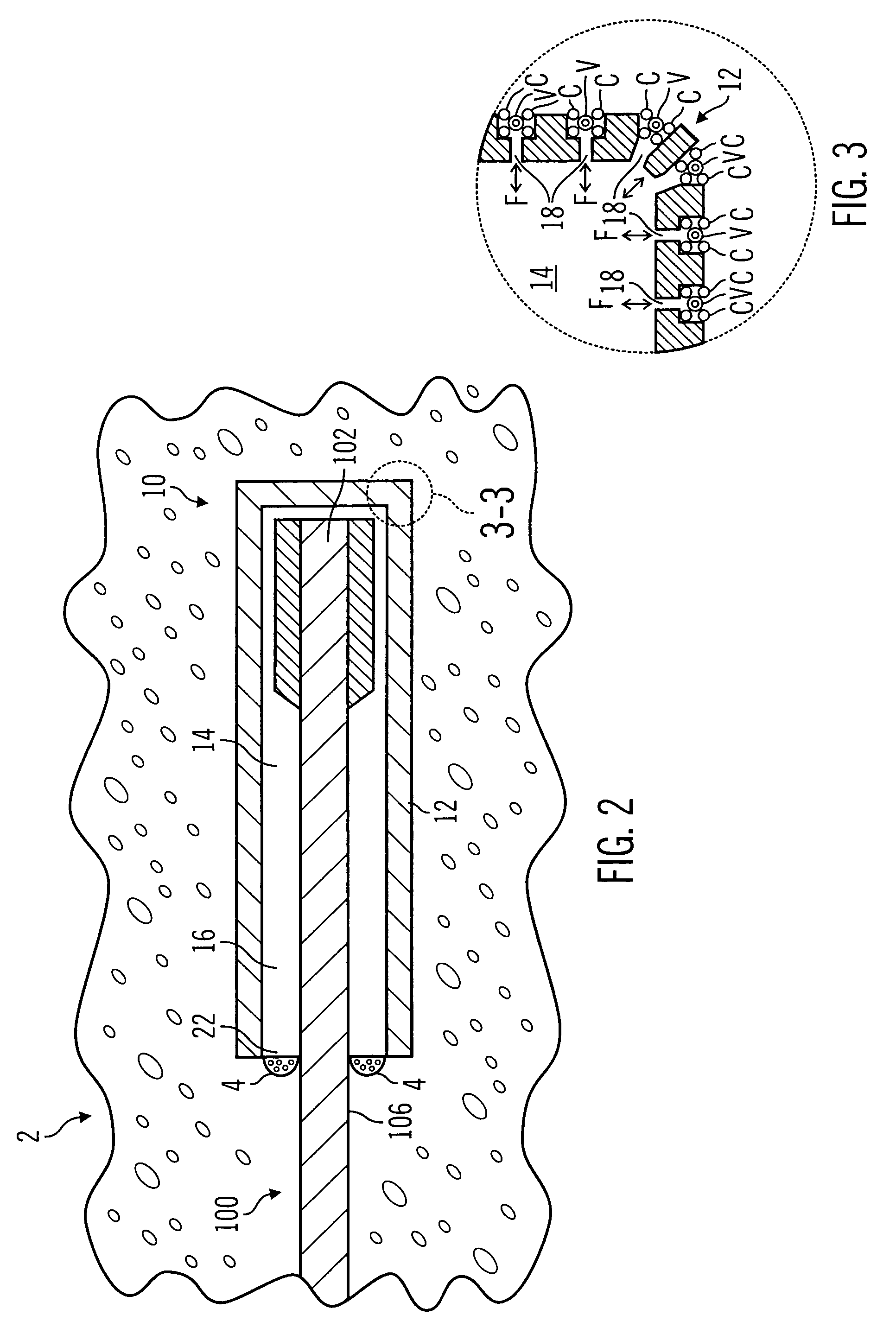
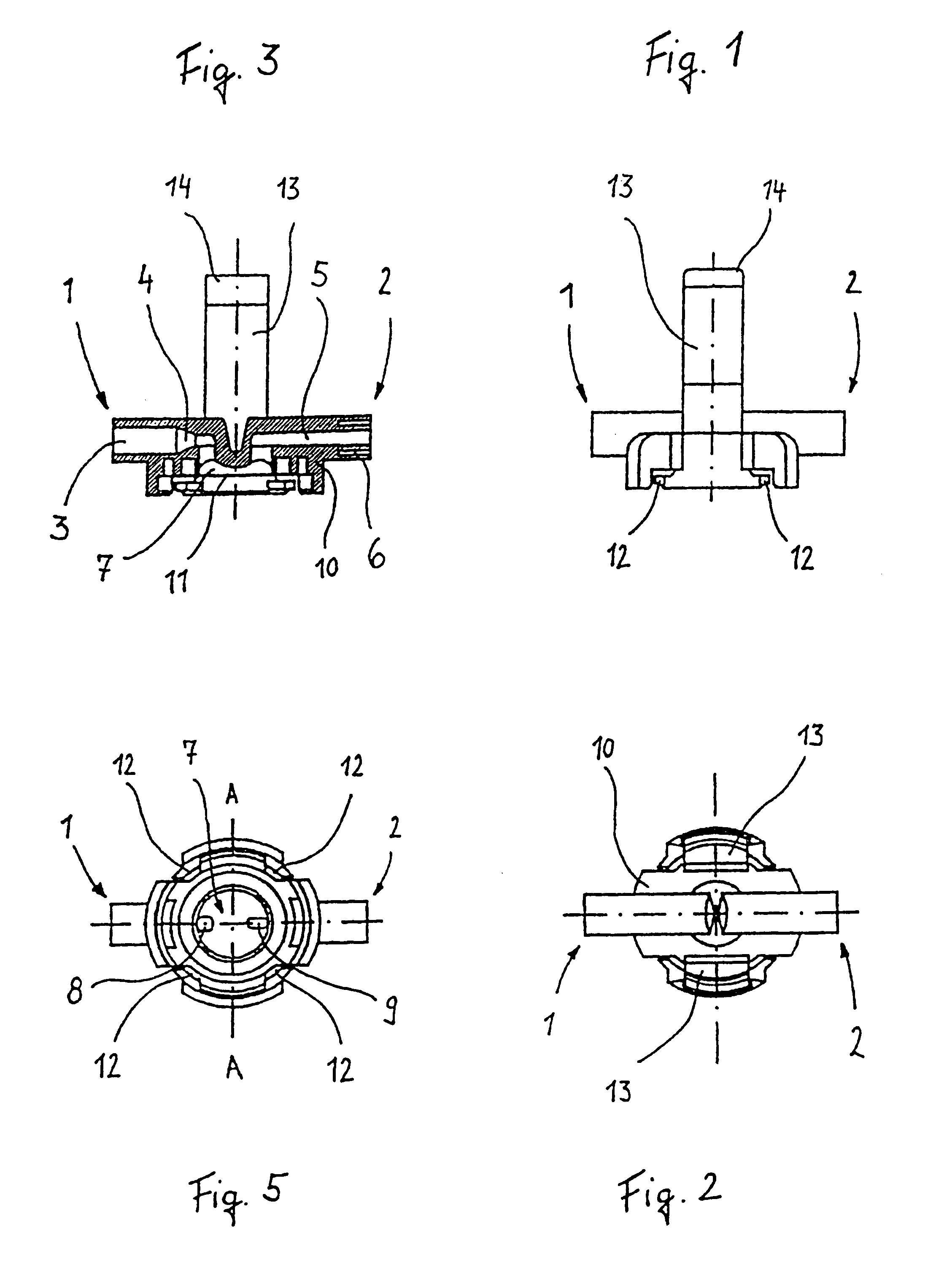
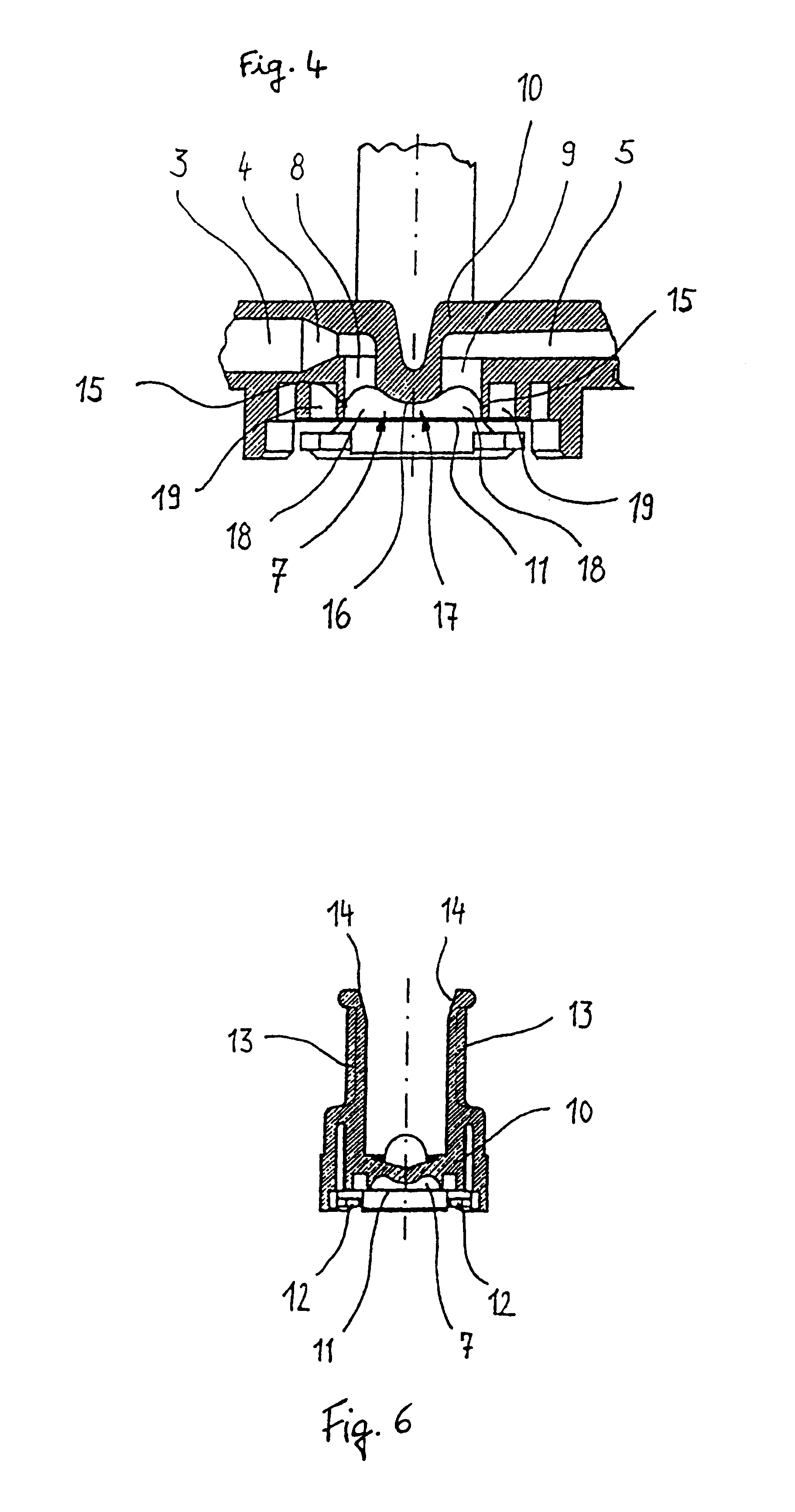
A surgical sealing device
A surgical sealing device (405), suitable for use during a surgical procedure such as a laparoscopic procedure or a hand-assisted laparoscopic procedure, comprises a first sealing member (5) and a second sealing member (6). A thin slit is defined between the sealing members (5, 6). In a closed configuration, the first sealing member (5) overlaps the distal end opening of a passageway (2) through the second sealing member (6) to prevent leakage of insufflation gases out of an abdomen (4). In an open configuration, the first sealing member (5) is retracted to reveal the distal end opening of the passageway (2) and thus facilitate passage of an object, such as a surgeon's hand or forearm (3), into the abdomen (4). The device (405) comprises a planar support element (406) fixedly attached to the proximal exterior surface (408) of the first sealing member (5). The first sealing member (5) is fixedly attached to the second sealing member (6) at an attached region (409), with a detached region (407) remaining detached from the second sealing member (6). The support element (406) acts as a stiffening element to increase the stiffness of the detached region (407) of the first sealing member (5) to minimise the deformation of the detached region (407) of the first sealing member (5) when the surgeon's hand / forearm (3) is pushed through the passageway (2). It is thus easier for the surgeon to retract the first sealing member (5) laterally to reveal the distal end opening of the passageway (2) by pushing the hand / forearm (3) through the passageway (2) and easier for the surgeon to pass the hand / forearm (3) through the device (405) and gain access to the abdomen (4).
Owner:ATROPOS LTD
Reusable analyte sensor site and method of using the same
A reusable analyte sensor site for use with a replaceable analyte sensor for determining a level of an analyte includes a site housing and a resealable insertion site coupled to one end of the site housing. Preferably, the site housing is formed to have an interior cavity, and includes an outer membrane made of a material selected to promote vascularization and having a first pore size, and an inner membrane made of a material selected to be free of tissue ingress. Also, the site housing permits the analyte to pass through the site housing to the interior cavity to permit measurement by the replaceable analyte sensor. The resealable insertion site is provided for inserting the replaceable analyte sensor into the interior cavity of the site housing.
Owner:MEDTRONIC MIMIMED INC
Intralumenal material removal using a cutting device for differential cutting
InactiveUS7344546B2Minimal damageFacilitates translation and navigationUltrasonic/sonic/infrasonic diagnosticsCannulasDrive shaftMaterial removal
Intralumenal material removal systems are provided using an advanceable and rotatable cutter assembly designed for differential cutting. The intralumenal material removal system includes a cutter assembly positionable in the body cavity of a mammalian subject. One embodiment of the cutter assembly includes a cutter with blades that are designed and arranged to form an acute blade angle of attack with the matter-to-be-removed. The cutter assembly is axially advanceable by translating the drive shaft and rotatable by rotating the drive shaft. The occlusive material is scraped by the cutter assembly and may be aspirated to remove the material from the body cavity. The cutter assembly may provide aspiration ports positioned between facing surfaces of the blades.
Owner:BOSTON SCI LTD
Needleless valve
A needless valve is described which avoids the suctioning problems of the prior needleless devices upon deactivation and which preferably provides a positive self-purging effect. The valve is self-purging at the end of an administration cycle, avoiding clogging of attached catheters or other devices, and ensures that substantially all of liquid received into the valve is delivered to the receiver. The valve is also extremely simple in design and easy to construct and assemble, since it consists of only three pieces. The valve has a base with a connector for fluid communication attachment to tubing or other device, a solid elongated fluid channeling rod, and an internal fluid flow conduit; a flexible hollow expandable and contractible plug fitting over and moveable along the rod; and a tubular housing fitting over the plug and attached to the base. When the valve is activated by insertion of a nozzle of a fluid source, the rod and plug wall cooperate so that as the plug retracts along the rod, it is stretched and its interior expanded. Upon deactivation, the plug contracts, the interior volume decreases, and the resiling plug wall forces residual fliud within the valve to be expelled through the outlet, purging the residual fluid from the valve. No negative pressure is formed, so no suctioning of blood or other fluid from a patient or receiver occurs.
Owner:CAREFUSION 303 INC
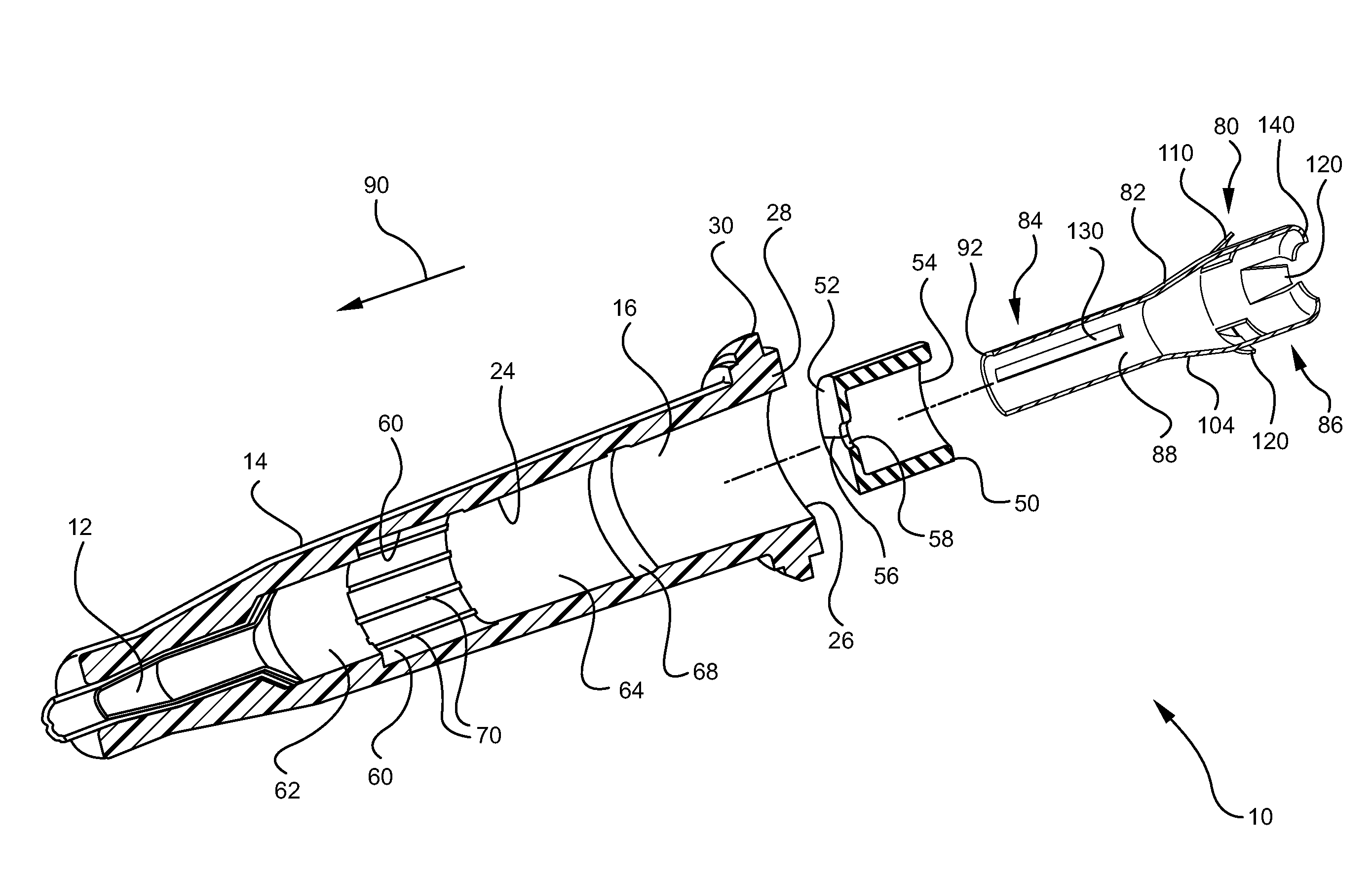
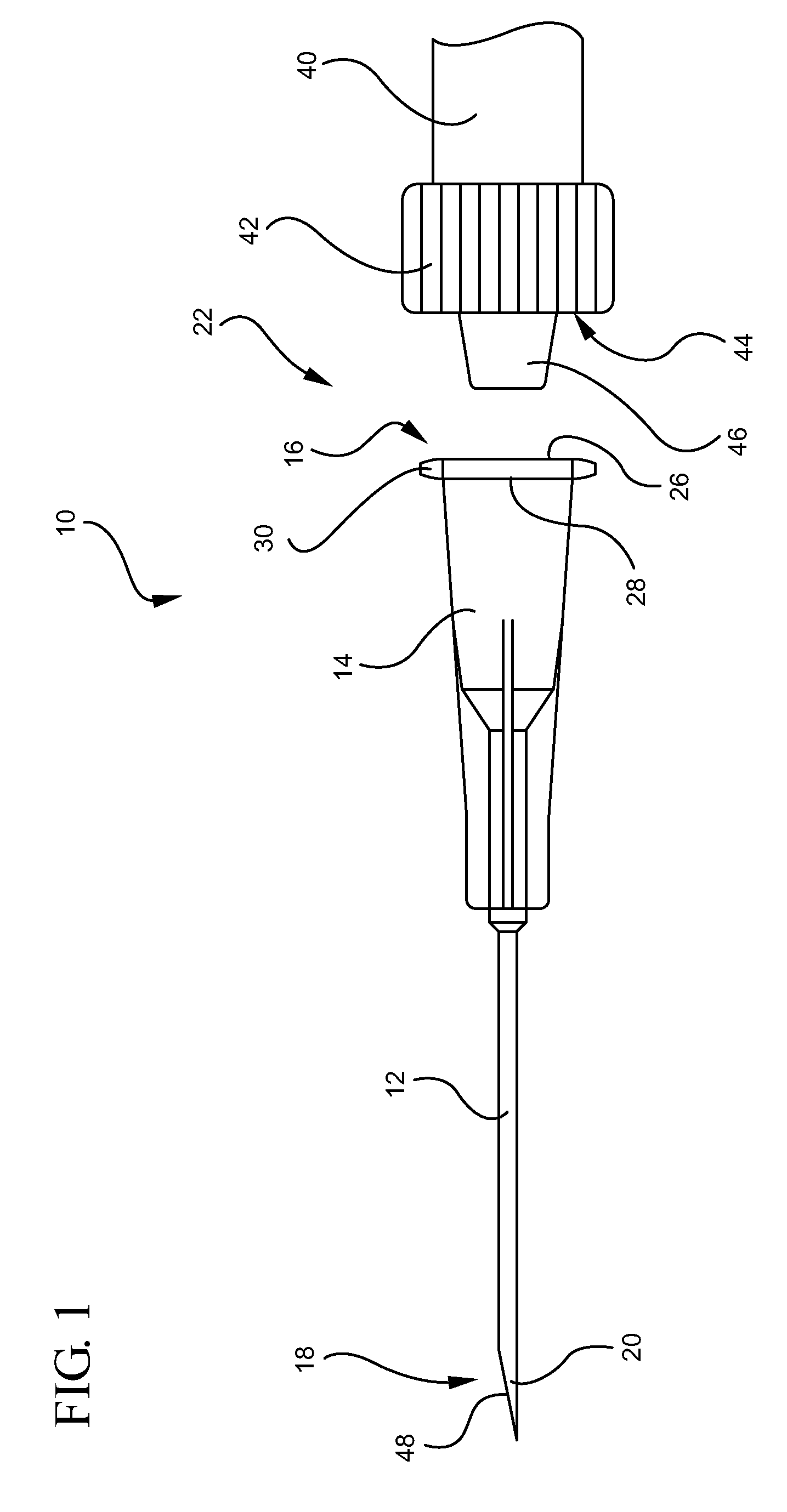
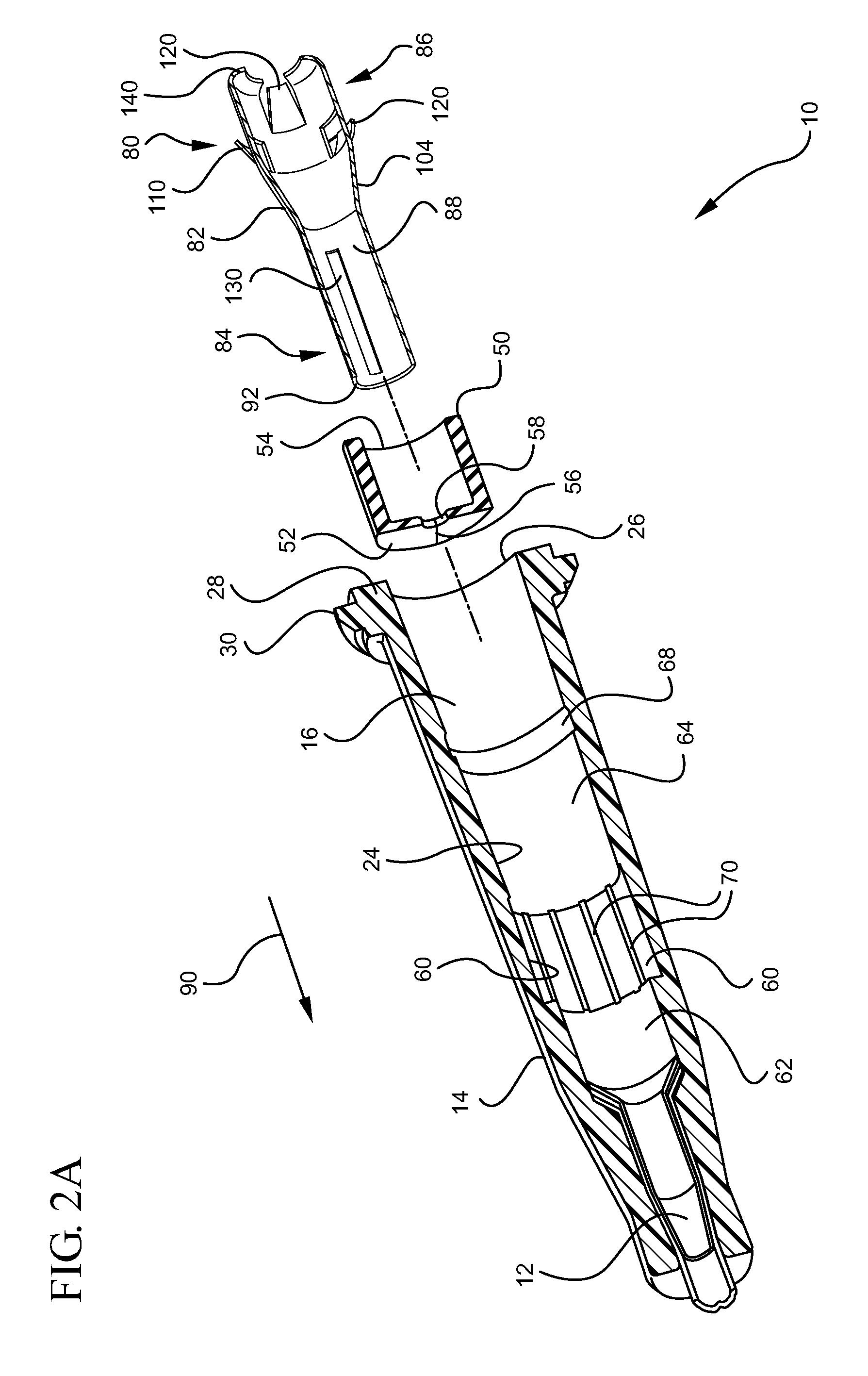
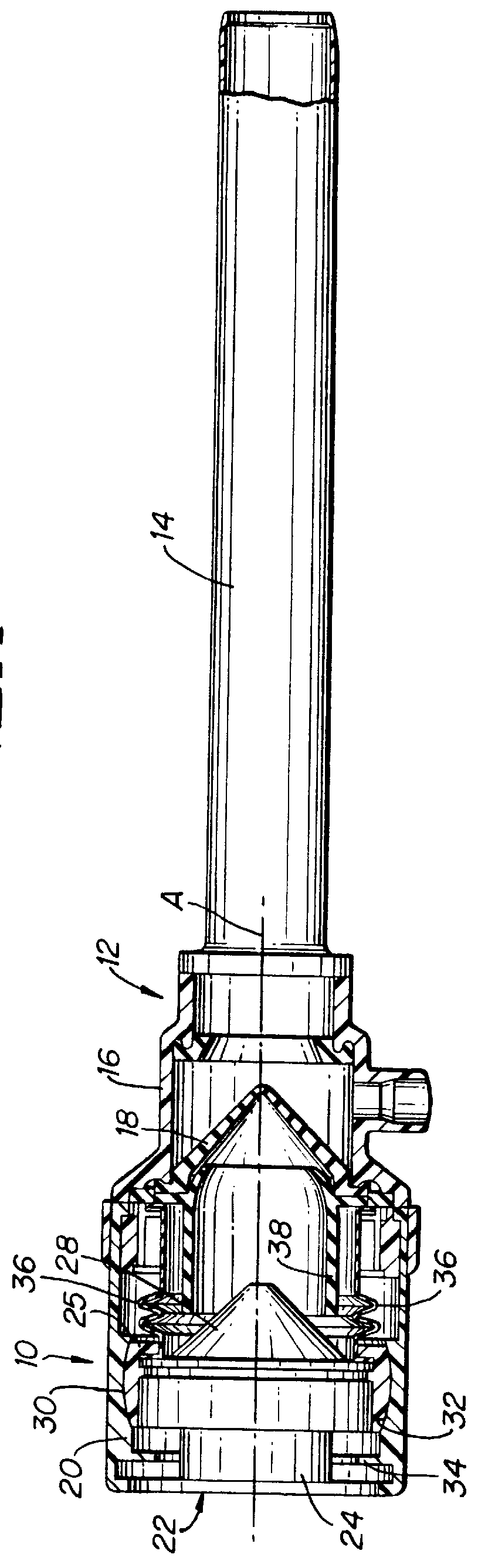
Ported iv catheter having external needle shield and internal blood control septum
An extravascular system is provided which includes a catheter adapter having a blood control septum configured to control flow of a fluid through the catheter adapter, the catheter adapter further having a catheter configured for intravenous insertion. The extravascular system further includes a septum activator slidably inserted within the catheter adapter and configured for advancement through the blood control septum to provide a fluid pathway through the blood control septum. Further still, the extravascular system includes an external safety mechanism comprising a needle hub and a needle shield interconnected via a tether, wherein the needle shield includes a safety clip that is configured to retain a sharpened end of the introducer needle within the needle shield. Some implementations further comprise an access port forming a portion of the catheter adapter and providing selective access to the lumen of the catheter adapter.
Owner:BECTON DICKINSON & CO
Splittable medical valve
InactiveUS7101353B2Readily pulled apartEasy to separateValve arrangementsCannulasMedicineDistal portion
A hemostatic valve couples to a sheath proximal hub having an inside and an outside surface. The hemostatic valve has a valve body having a proximal porting and end, and a distal portion and end. A passageway extends longitudinally through the valve body between the proximal and distal ends. A sealing element is positioned within the passageway. The distal portion of the valve body includes a pair of contact surfaces forming an annular gap receiving the sheath proximal hub of the sheath so that the contact surfaces sandwich the sheath proximal hub forming a seal on both the inside and outside surfaces of the hub. The valve body and the sheath proximal hub includes two oppositely placed longitudinal lines of fissure that allow the hub to be separated into two halves with the valve body.
Owner:COOK MEDICAL TECH LLC
Cardiovascular sheath/catheter
InactiveUS6595959B1Convenient treatmentFacilitate studyGuide needlesInfusion syringesVascular diseaseDilator
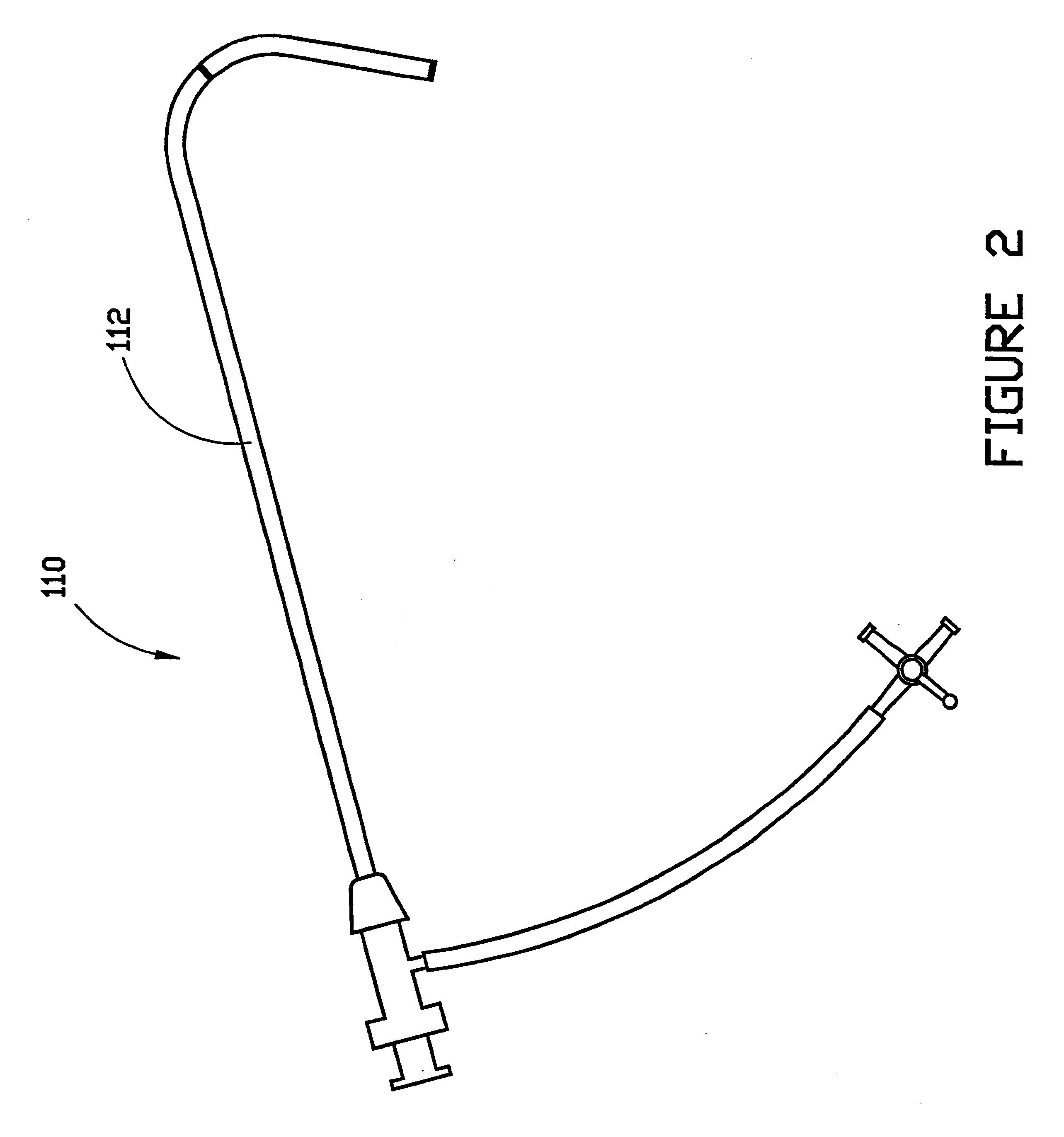
A vascular interventional device may be introduced over a guidewire into a vessel of the cardiovascular system of a patient. This device includes a hollow, flexible tube having a proximal end and a distal end that is adapted to selectively engage a target vessel of the cardiovascular system of the patient. This tube also includes a lumen that is continuous from the proximal to the distal end, and has an end hole in the distal end that is in fluid communication with the lumen. The device also includes a hollow vessel dilator that is adapted for insertion into and through the tube and over the guidewire. The dilator has an inside diameter that is slightly larger than the guidewire and an outside diameter that is slightly smaller than the diameter of the lumen of the tube. The distal end of the dilator is adapted to accommodate vascular entry over the guidewire, and the dilator is adapted to dilate the vessel to accept the tube. The device also includes a hub at the proximal end of the tube. The hub includes an end port through which a second interventional device having an outside diameter smaller than the diameter of the lumen may be introduced into the lumen of the tube. The hub also includes a side port through which a fluid agent may be injected for delivery through the lumen and out the end hole of the tube. A sealing mechanism is also provided in the hub to prevent air from entering the tube and blood and other fluids from leaking out of the tube through the hub. A pair of vascular interventional devices, at least one of which is constructed according to the invention, may be utilized to treat or study a cardiovascular condition, or to measure the blood pressure across a vascular segment.
Owner:STRATIENKO ALEXANDER A
Cryotreatment device and method
InactiveUS7220257B1Reduce adverse reactionsReduced responseStentsOther blood circulation devicesCoronary artery angioplastyPercent Diameter Stenosis
Devices and methods for cooling vessel walls to inhibit restenosis in conjunction with medical procedures such as coronary artery angioplasty. Stenosed vessel walls can be cooled prior to angioplasty, after angioplasty, or both. The invention is believed to inhibit restenosis through cooling to a temperature near freezing, preferably without causing substantial vessel wall cell death. One catheter device includes a distal tube region having coolant delivery holes radially and longitudinally distributed along the distal region. In some devices, holes spray coolant directly onto the vessel walls, with the coolant absorbed into the blood stream. In other embodiments, a balloon or envelope is interposed between the coolant and the vessel walls and the coolant returned out of the catheter through a coolant return lumen. Some direct spray devices include an occlusion device to restrict blood flow past the region being cooled. Pressure, temperature, and ultrasonic probes are included in some cooling catheters. Pressure control valves are included in some devices to regulate balloon interior pressure within acceptable limits. In applications using liquid carbon dioxide as coolant, the balloon interior pressure can be maintained above the triple point of carbon dioxide to inhibit dry ice formation. Some cooling catheters are coiled perfusion catheters supporting longer cooling periods by allowing perfusing blood flow simultaneously with vessel wall cooling. One coiled catheter is biased to assume a coiled shape when unconstrained and can be introduced into the body in a relatively straight shape, having a stiffening wire inserted through the coil strands.
Owner:BOSTON SCI SCIMED INC
Systems and methods for providing a flushable catheter assembly
ActiveUS20110046570A1Maintain positionPrevent of positive pressureGlovesPretreated surfacesCATHETER ADAPTERBiological activation
A flushable catheter assembly having features to enable selective activation of fluid flow through the catheter assembly is disclosed herein. A septum is placed within the catheter adapter of the catheter assembly and includes a pathway that is closed prior to being biased open via a septum activator also positioned within the catheter adapter. A plurality of air vent channels is interposed between the septum and the inner surface of the catheter adapter to permit “flashback” of blood during insertion of the catheter into a patient. The septum activator is advanced through the pathway of the septum as a coupler is attached to a proximal opening of the catheter adapter.
Owner:BECTON DICKINSON & CO
Seal assembly for accommodating introduction of surgical instruments
InactiveUSRE36702E1Facilitates seal maintenanceEasy alignmentCannulasSurgical needlesEndoscopic surgeryEngineering
A seal assembly is provided which includes a gimbal-like structure permitting rotation of a mounting member relative to the housing. The rotation of the mounting member allows the seal member to align with an instrument being inserted therethrough. The seal assembly is adapted to be detachably mounted to a cannula assembly for use in endoscopic surgery.
Owner:UNITED STATES SURGICAL CORP
Safety catheter system and method
A catheter integral with a valved needle-free connector provides a safety catheter device configured to receive a blunt cannula and sharp needle forming an insertion mechanism. The sharp needle is mounted within a needle tube and a control handle is used to slide the sharp needle out of and into the protective needle tube. When the insertion mechanism is mounted to the connector and the control handle is used to slide the sharp needle out of the tube, a blunt cannula first moves into contact with and enters the bore of the valve mechanism of the connector opening it and protecting it from damage that may be caused by the sharp needle. The sharp needle is then extended through the connector and extends out the distal end of the catheter so that a venipuncture procedure may be performed to properly locate the catheter in the patient's circulatory system. Once located, the needle may be retracted into the insertion mechanism, the insertion mechanism disconnected from the connector, and discarded.
Owner:CAREFUSION 303 INC
Laparoscopic instrument and cannula assembly and related surgical method
Owner:TYCO HEALTHCARE GRP LP
System, Method, and Apparatus for Monitoring, Regulating, or Controlling Fluid Flow
ActiveUS20130310990A1Reduce internal volumeReduce liquid volumeImage analysisEngine diaphragmsEngineeringActuator
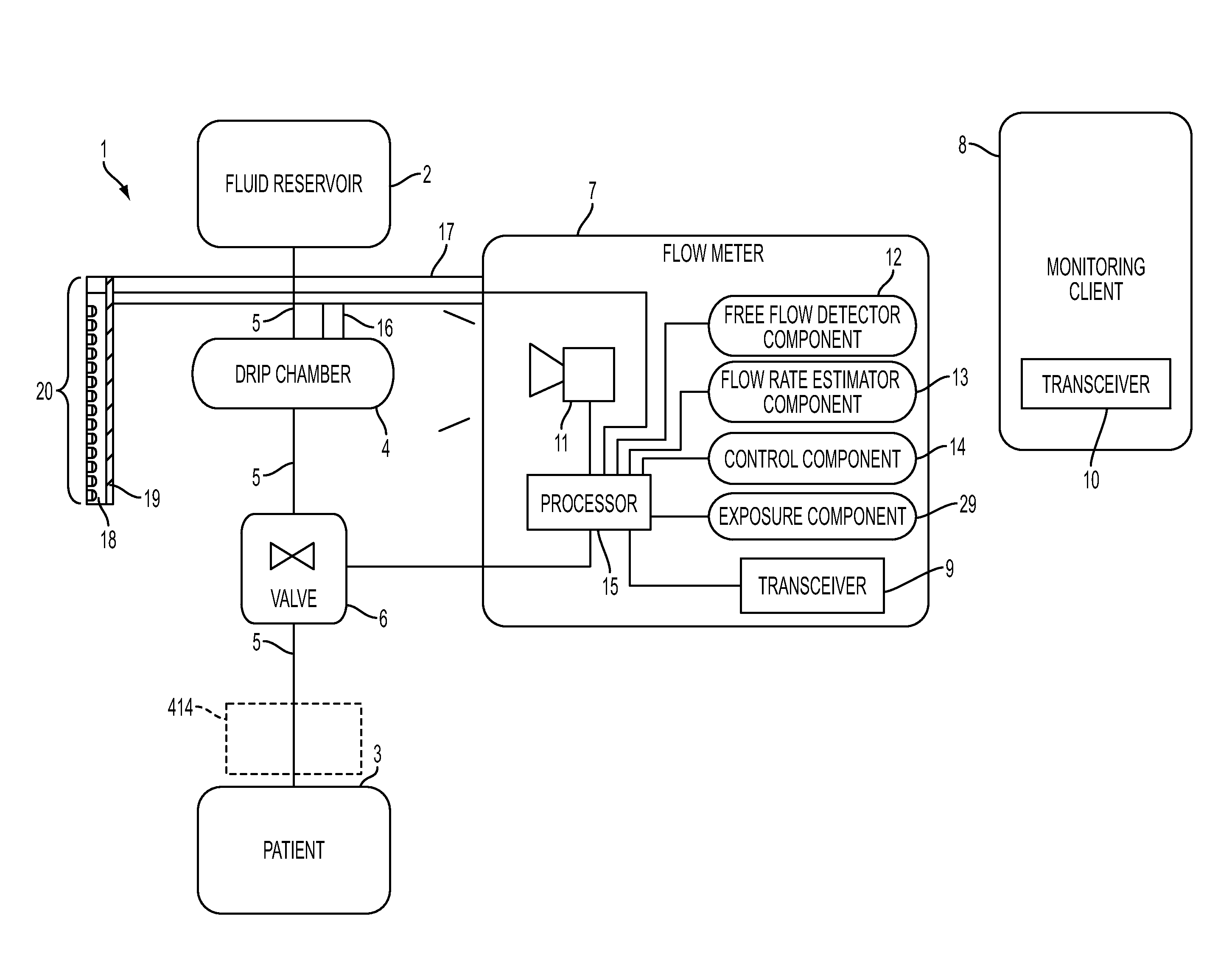
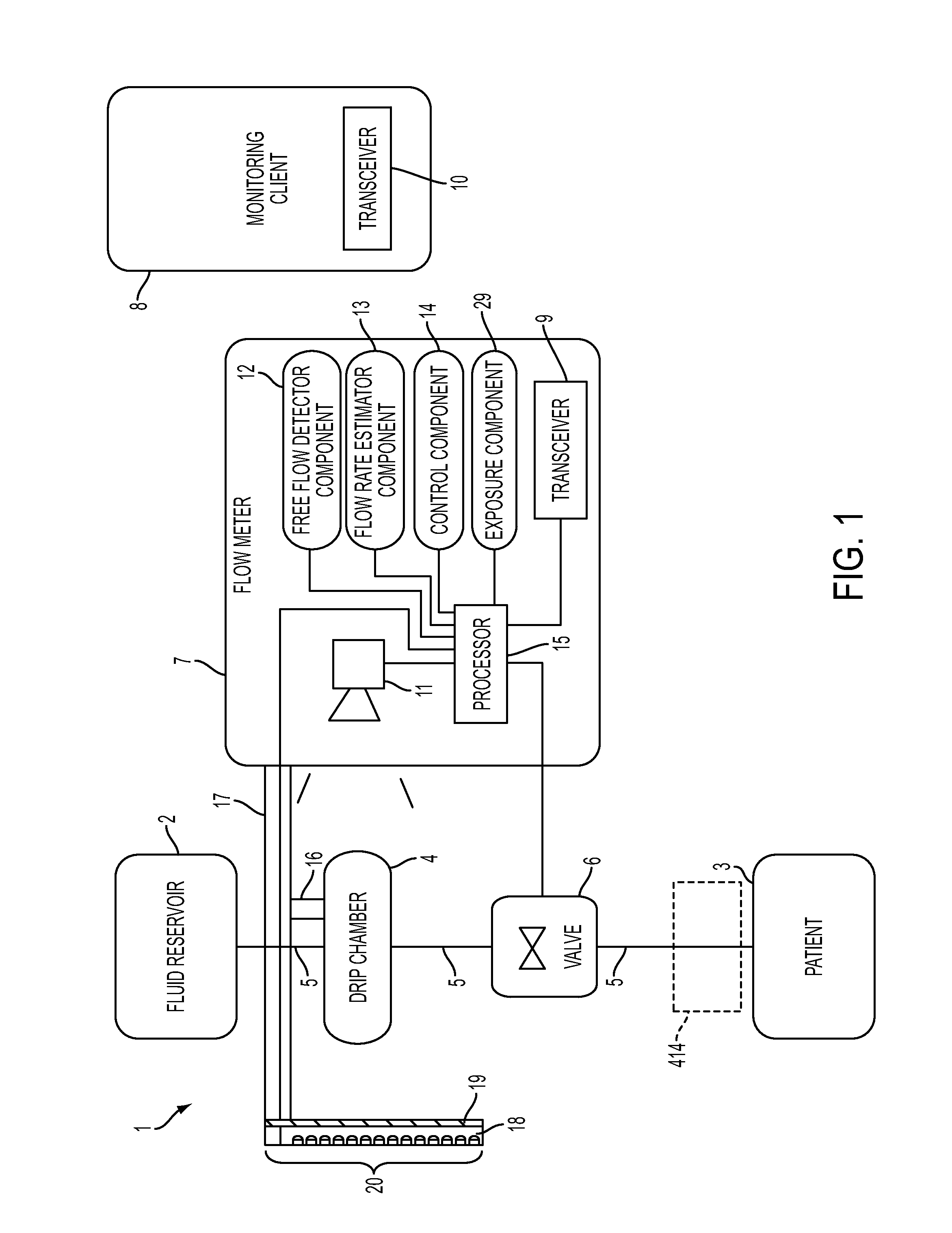
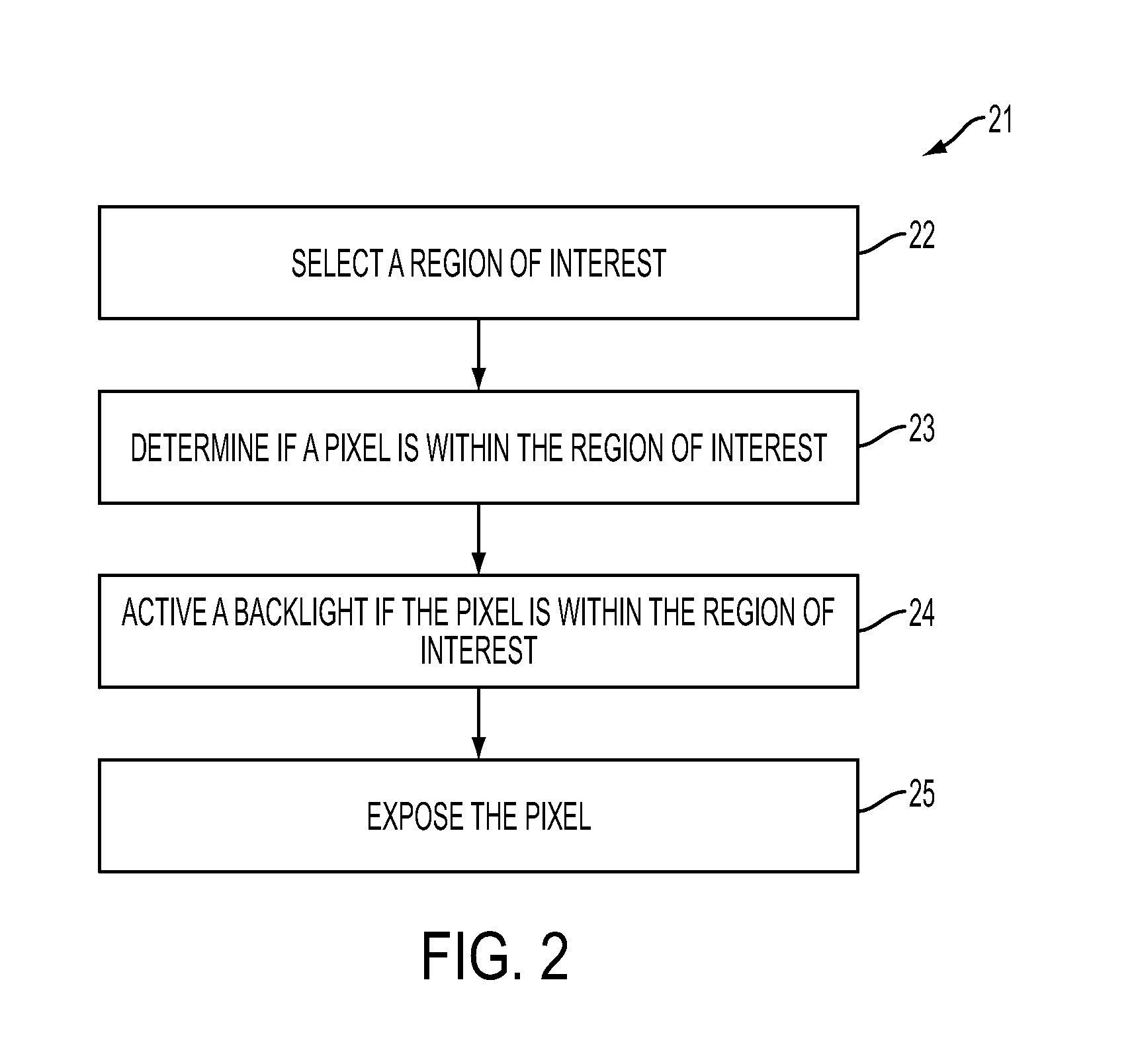
A flow meter, and related system and method are provided. The flow meter includes a coupler, a support member, an image sensor, a valve, and one or more processors. The coupler is adapted to couple to a drip chamber. The support member is operatively coupled to the coupler. The image sensor has a field of view and is operatively coupled to the support member. The image sensor is positioned to view the drip chamber within the field of view. The one or more processors are operatively coupled to the image sensor to receive image data therefrom and to the actuator to actuate the valve. The one or more processors are configured to estimate a flow of fluid through the drip chamber and to actuate the valve to control the flow of fluid through the drip chamber to achieve a target flow rate.
Owner:DEKA PROD LLP
Invisible needle
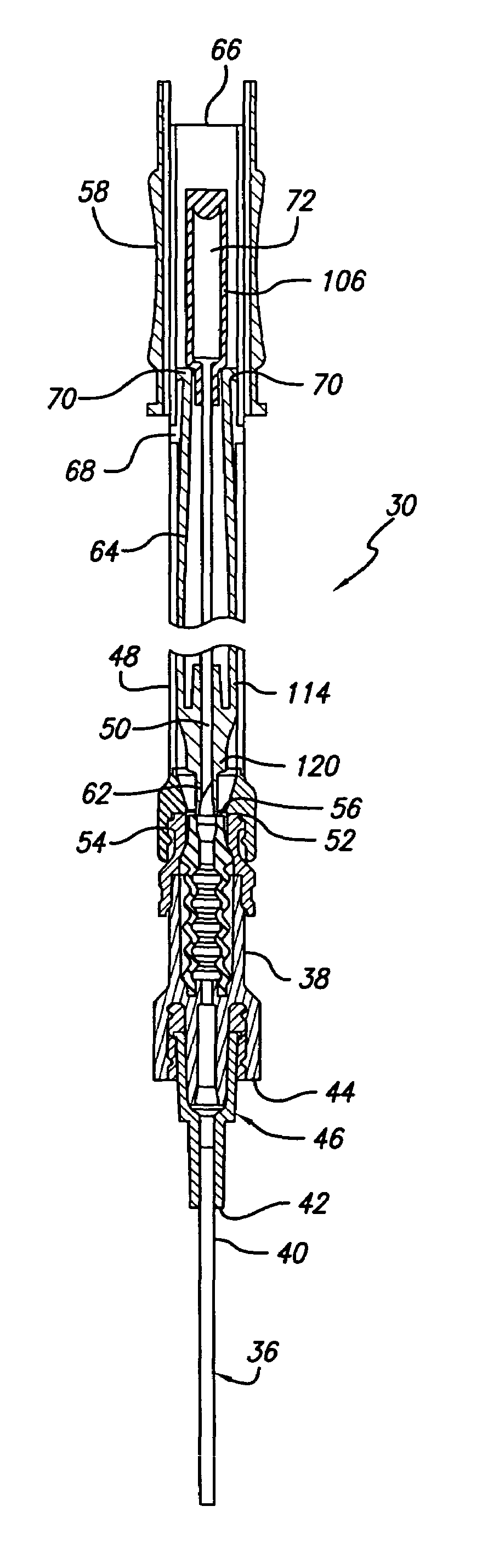
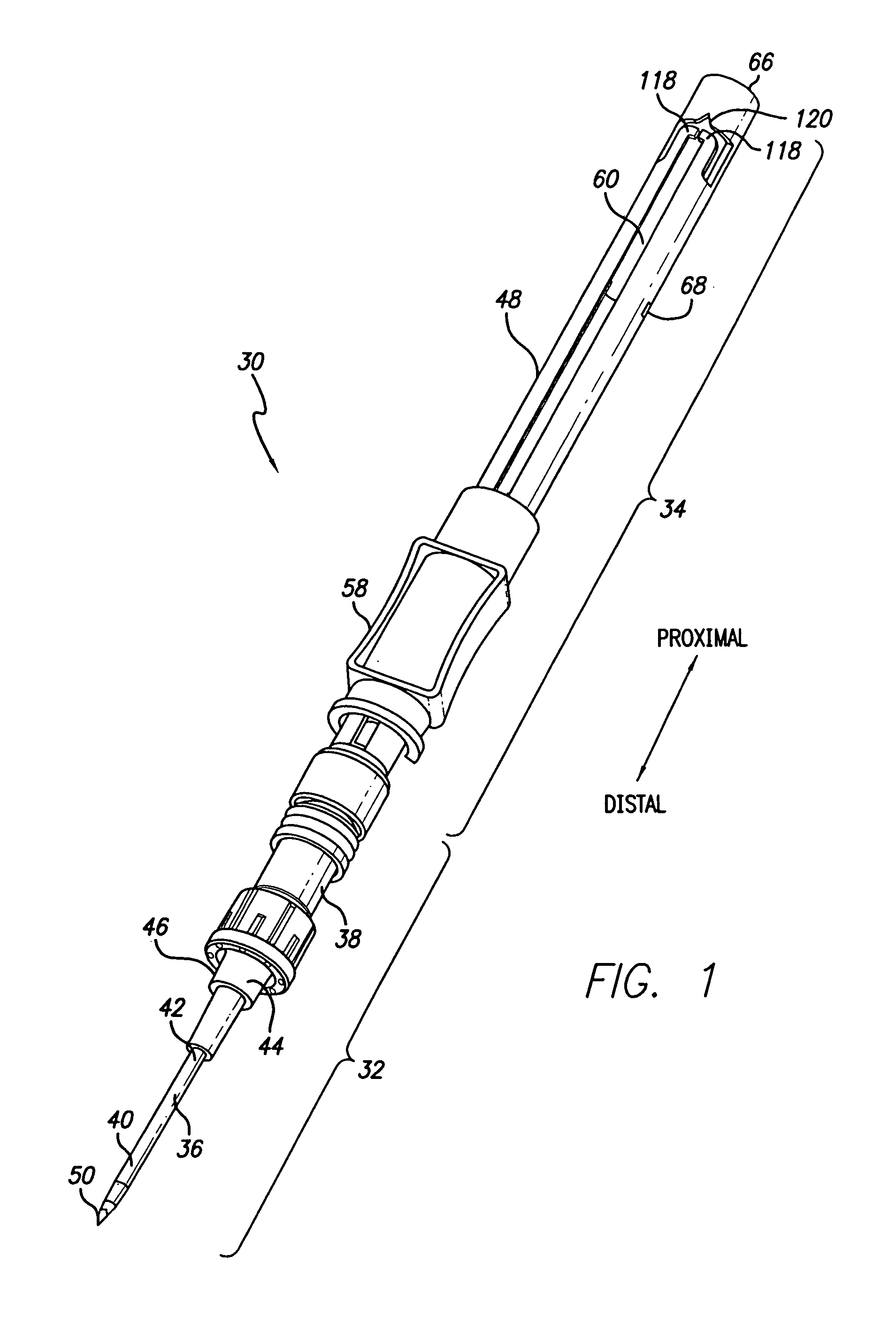
ActiveUS20070093754A1Simple and safe processNon-expensive inserterInfusion syringesCatheterInfusion setBiological activation
The invention relates to an inserter for an infusion set for intermittent or continuous administration of a therapeutical substance, such as e.g. insulin. The inserter comprises a needle hub comprising an insertion needle and two spring units assuring automatic insertion and automatic retraction of the insertion needle. The inserter comprises a housing (1), a carrier body (2) carrying an infusion part (8), a needle hub (3), a first moving unit (4) bringing the carrier body (2) to a forward position and a second moving unit (5) bringing the carrier body (2) to a retracted position. The inserter is characterized in that it has means for activation which should be activated at least once in order to bring the carrier body (2) from a retracted to a forward position, and back from the forward to the retracted position.
Owner:UNOMEDICAL AS
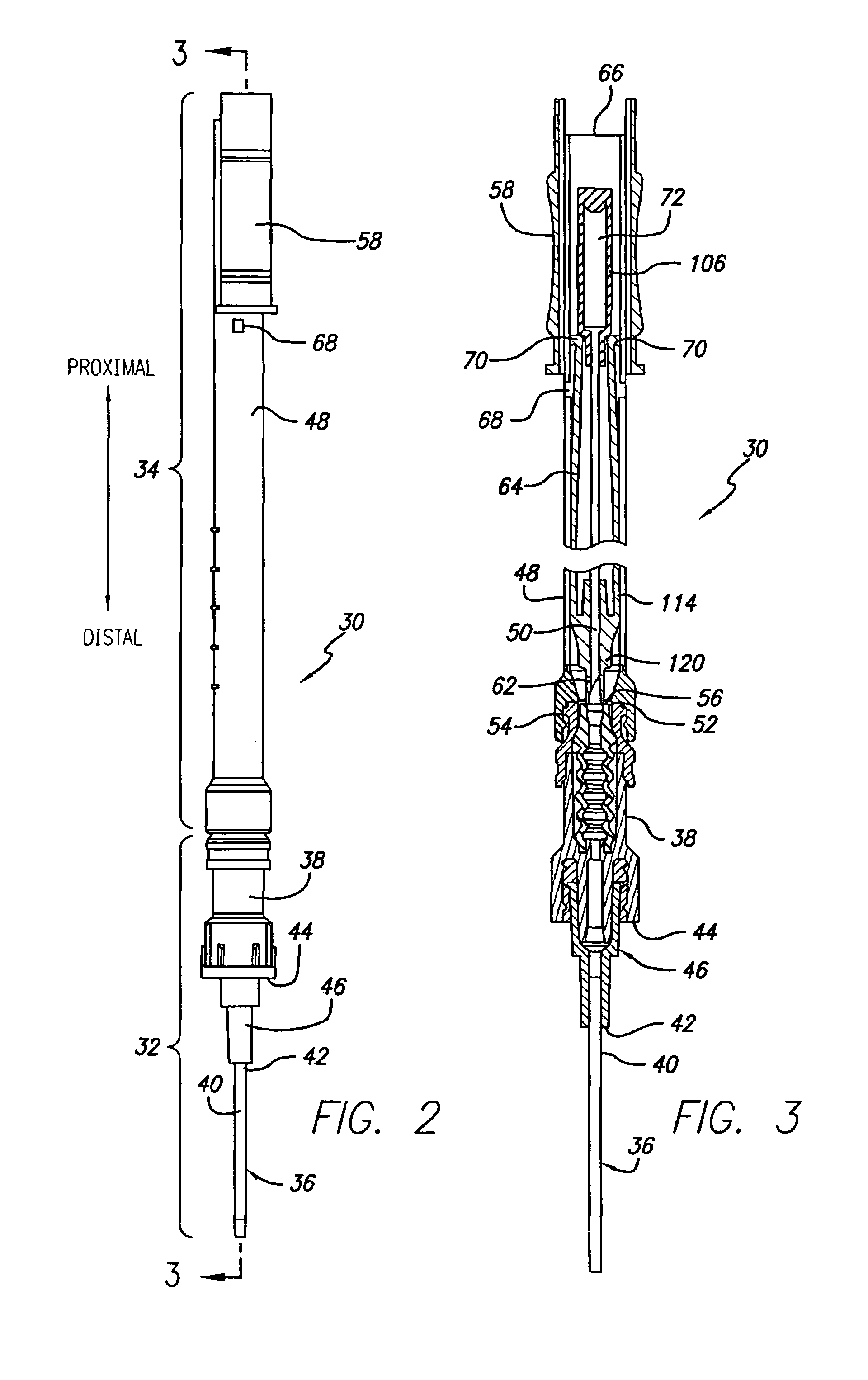
Mri-guided localization and/or lead placement systems, related methods, devices and computer program products
MRI compatible localization and / or guidance systems for facilitating placement of an interventional therapy and / or device in vivo include: (a) a mount adapted for fixation to a patient; (b) a targeting cannula with a lumen configured to attach to the mount so as to be able to controllably translate in at least three dimensions; and (c) an elongate probe configured to snugly slidably advance and retract in the targeting cannula lumen, the elongate probe comprising at least one of a stimulation or recording electrode. In operation, the targeting cannula can be aligned with a first trajectory and positionally adjusted to provide a desired internal access path to a target location with a corresponding trajectory for the elongate probe. Automated systems for determining an MR scan plane associated with a trajectory and for determining mount adjustments are also described.
Owner:CLEARPOINT NEURO INC
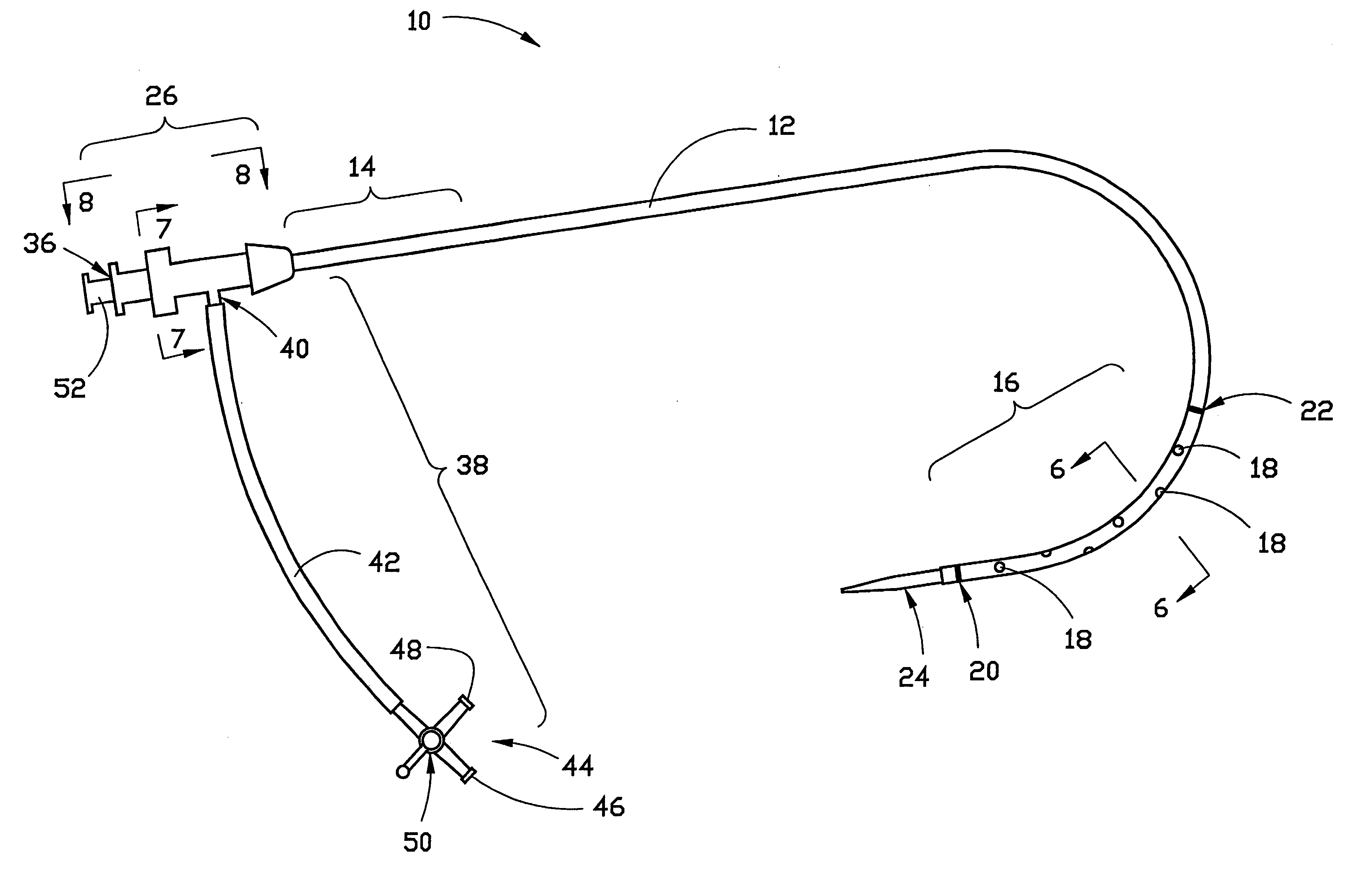
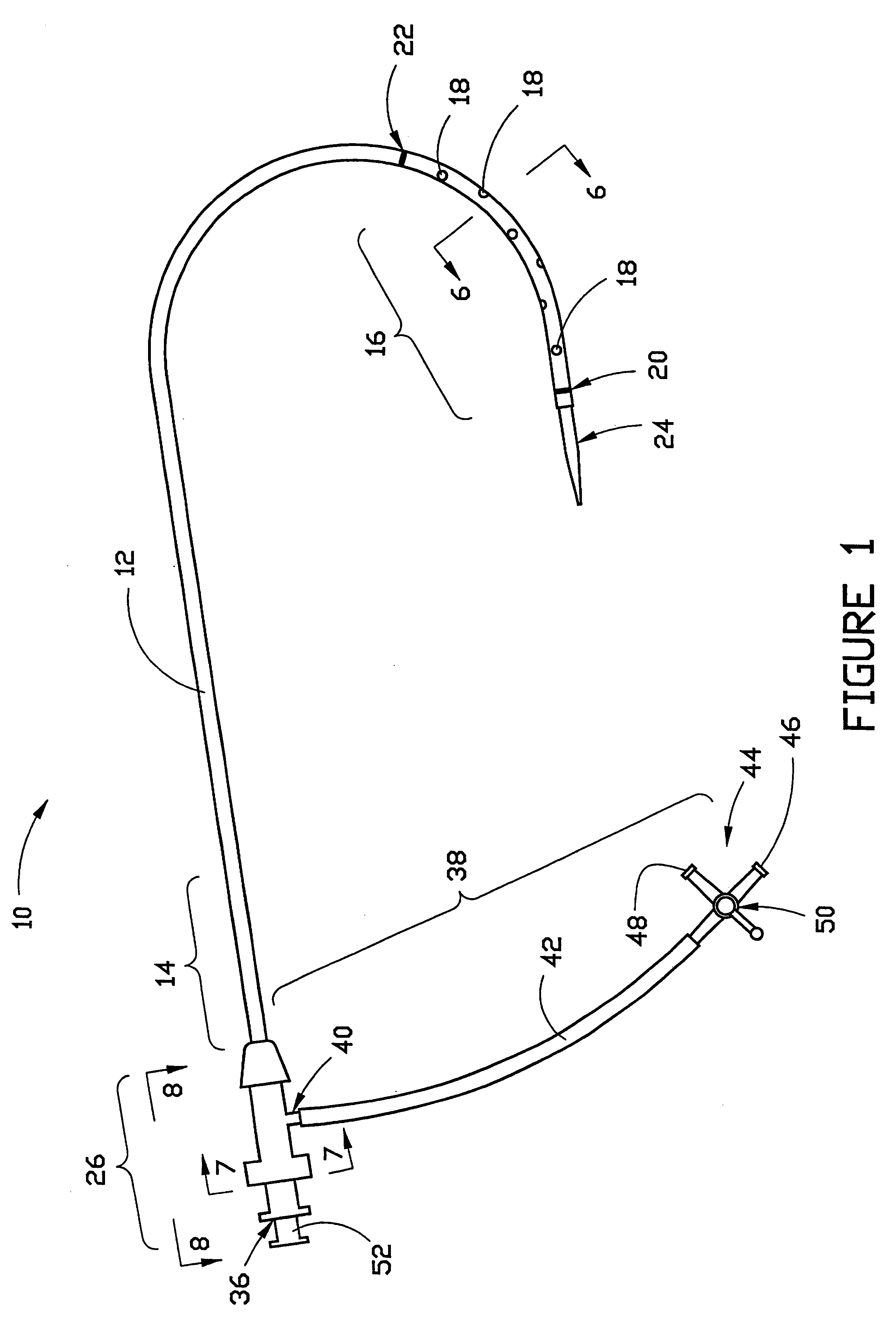
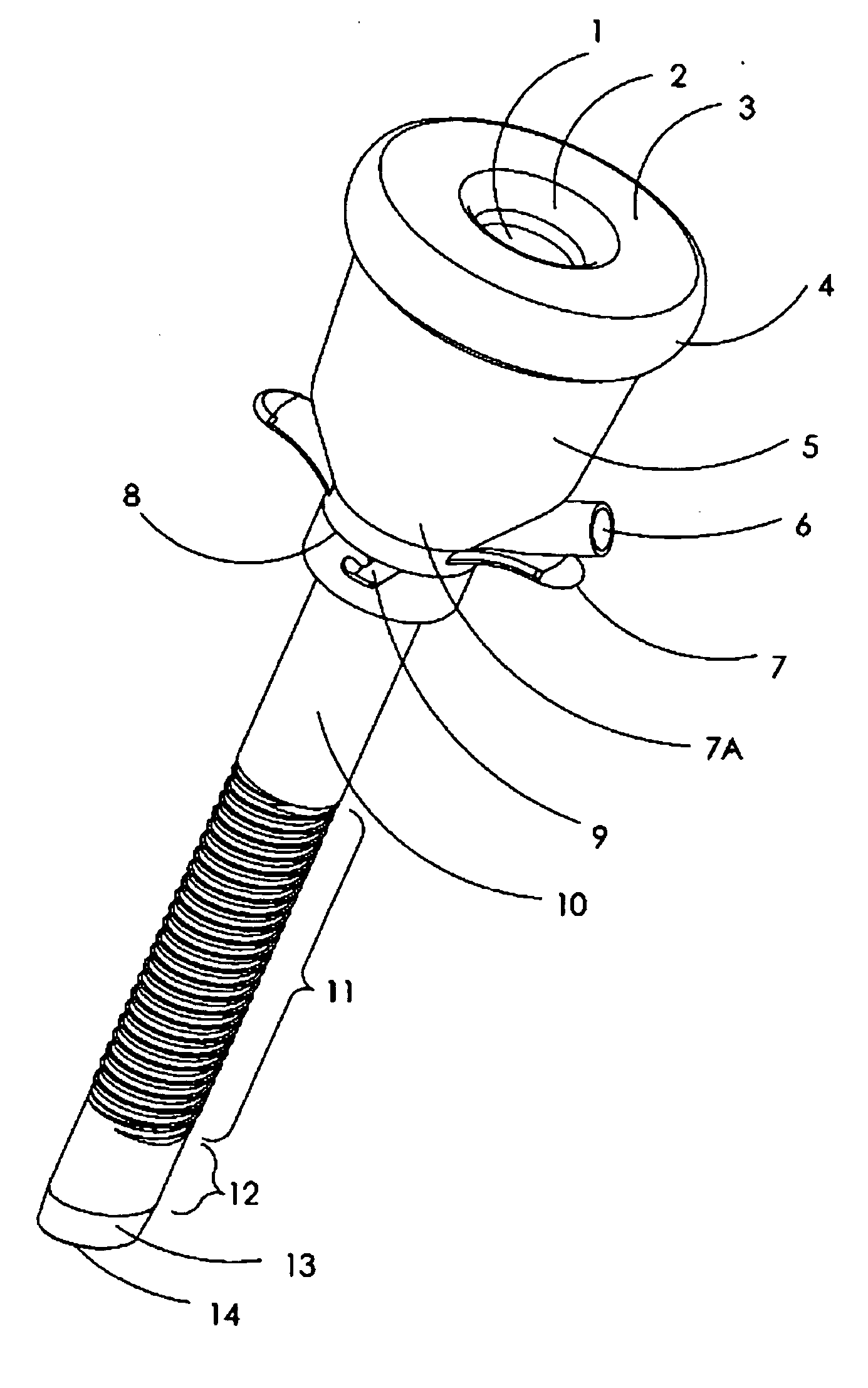
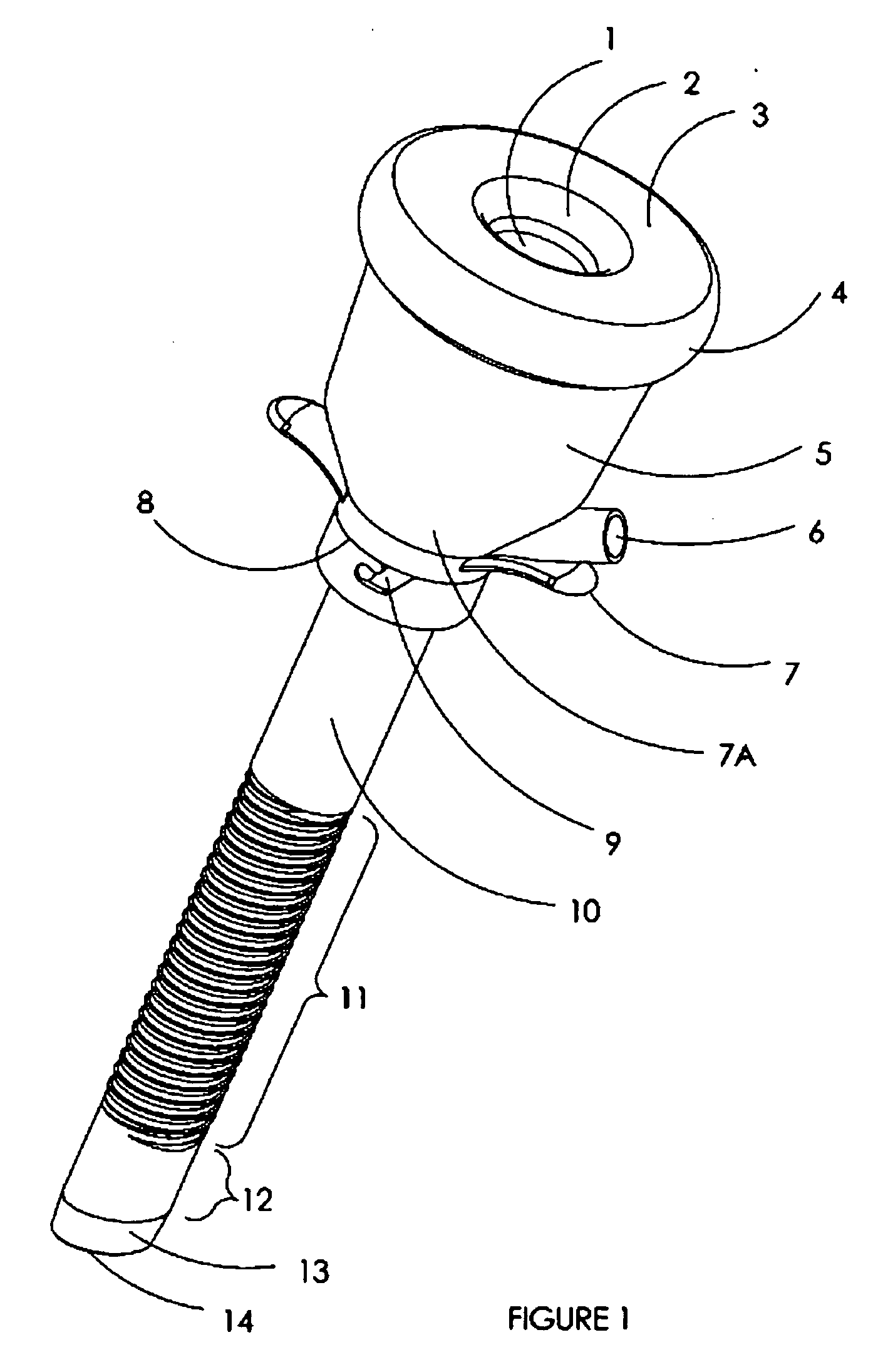
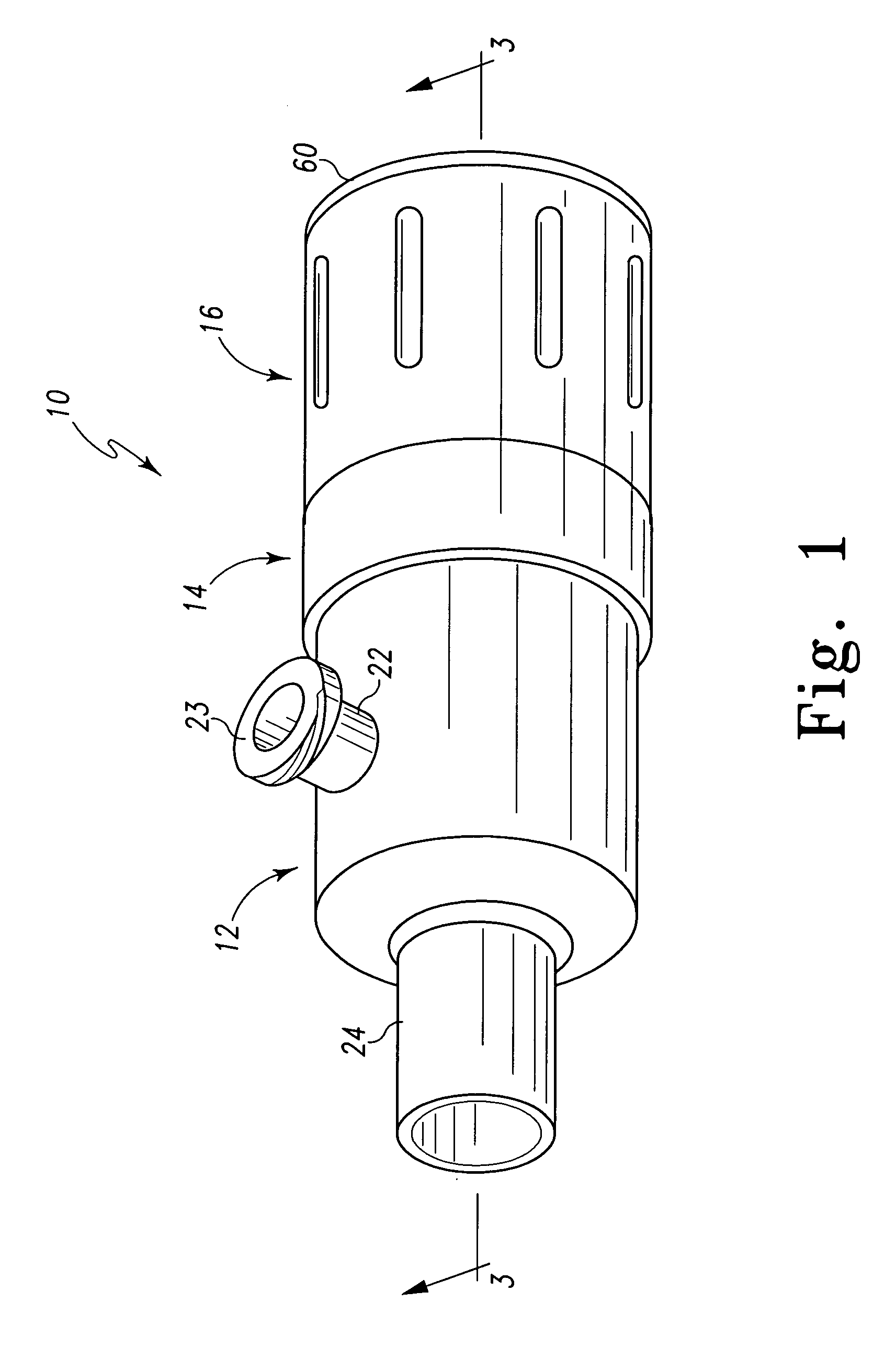
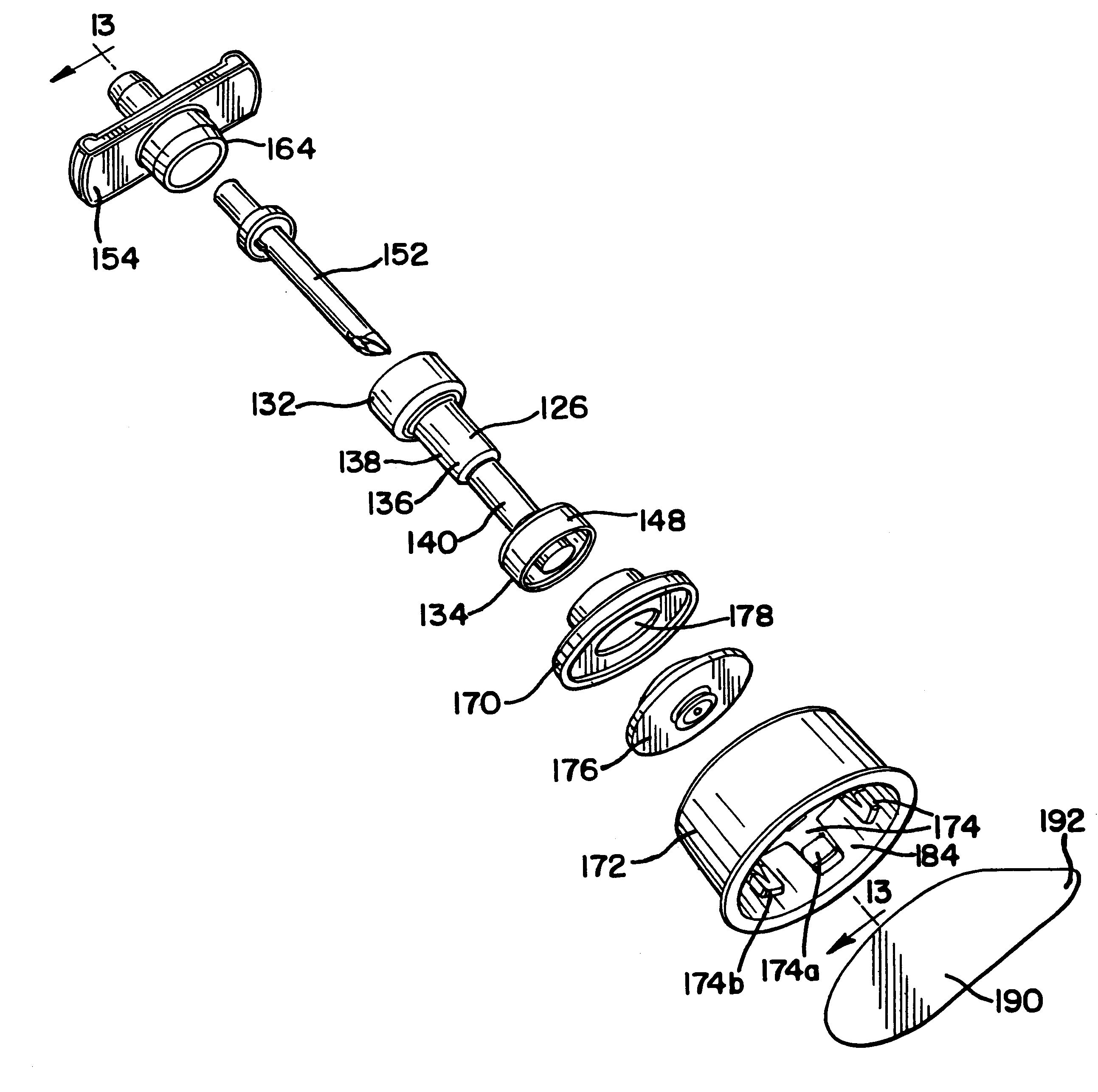
Sliding reconstitution device for a diluent container
The present invention provides a reconstitution device (10) for placing a first container (12), such as a liquid container (e.g. flexible container or syringe), in fluid communication with a second container (14), such as a drug vial. To this end, there is provided a connector device (10) for establishing fluid communication between the diluent container (12) and the drug vial (14). The connector device (10) has a first sleeve member (32) having a first end (36) and a second end (38). The first sleeve member (32) has at the first end (36), a first attaching member (30) adapted to attach to the liquid container (12). The connector device (10) also has a second sleeve member (34) having a first end (48) and a second end (50). The second sleeve member (34) is movable axially with respect to the first sleeve member (32) from an inactivated position to an activated position. The second sleeve member (34) has a protuberance (66) on an inner surface of the second sleeve member (34). A second attaching member (28) is attached on the second end (50) of the second sleeve member (34) and is adapted to attach to the second container (14). The second attaching member (28) has a sealing member (84). A piercing member (76) is positioned in the chamber and projects within the sleeve members (32,34) for providing a fluid flow path from the first container (12) to the second container (14). The piercing member (76) is moveable from a first position in the inactivated position to a second position in the activated position wherein the piercing member (76) moves past the protuberance (66), the protuberance (66) preventing movement of the piercing member (76) back to the first position. The device (10) is movable from the inactivated position to the activated position by a force generally applied to the device outside the liquid container (12).
Owner:BAXTER INT INC
Needleless valve
A needless valve is described which avoids the suctioning problems of the prior needleless devices upon deactivation and which preferably provides a positive self-purging effect. The valve is self-purging at the end of an administration cycle, avoiding clogging of attached catheters or other devices, and ensures that substantially all of liquid received into the valve is delivered to the receiver. The valve is also extremely simple in design and easy to construct and assemble, since it consists of only three pieces. The valve has a base with a connector for fluid communication attachment to tubing or other device, a solid elongated fluid channeling rod, and an internal fluid flow conduit; a flexible hollow expandable and contractible plug fitting over and moveable along the rod; and a tubular housing fitting over the plug and attached to the base. When the valve is activated by insertion of a nozzle of a fluid source, the rod and plug wall cooperate so that as the plug retracts along the rod, it is stretched and its interior expanded. Upon deactivation, the plug contracts, the interior volume decreases, and the resiling plug wall forces residual fluid within the valve to be expelled through the outlet, purging the residual fluid from the valve. No negative pressure is formed, so no suctioning of blood or other fluid from a patient or receiver occurs.
Owner:CAREFUSION 303 INC
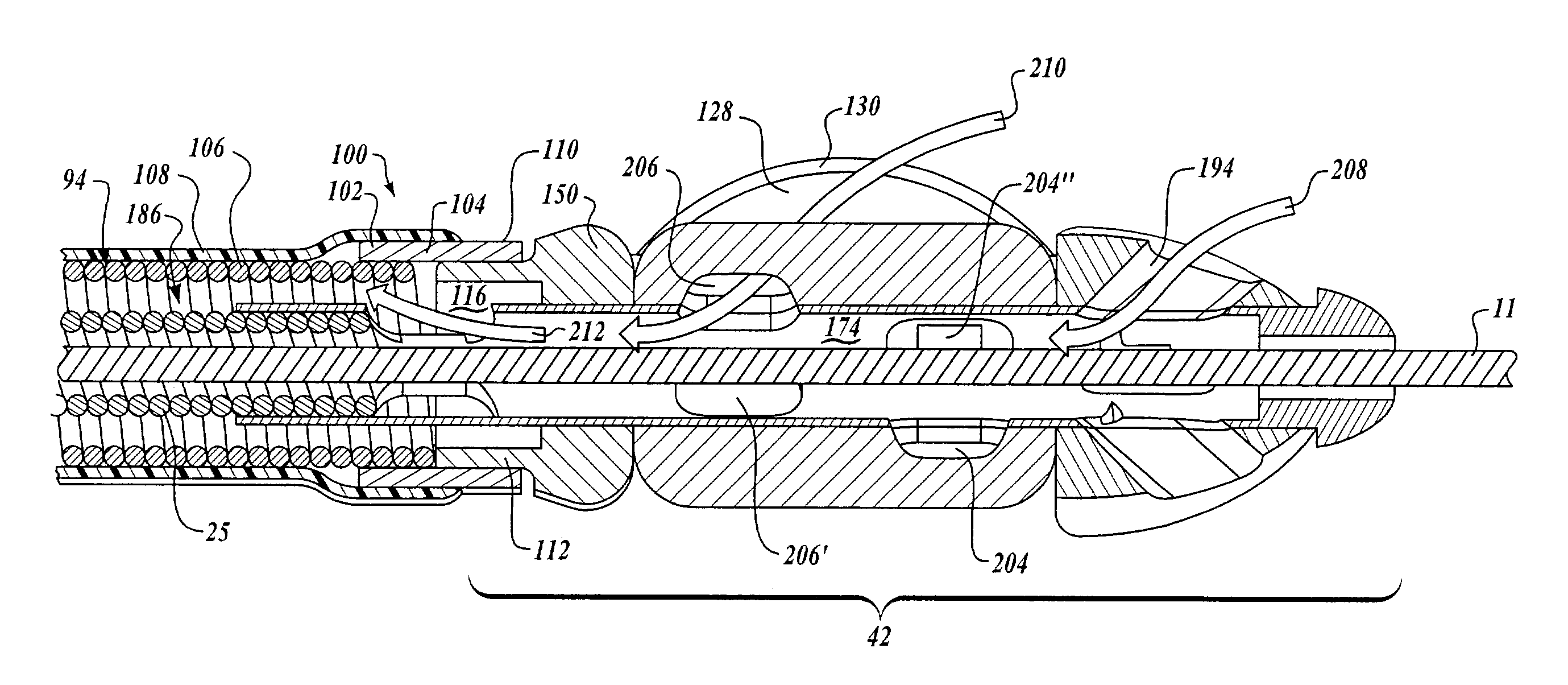
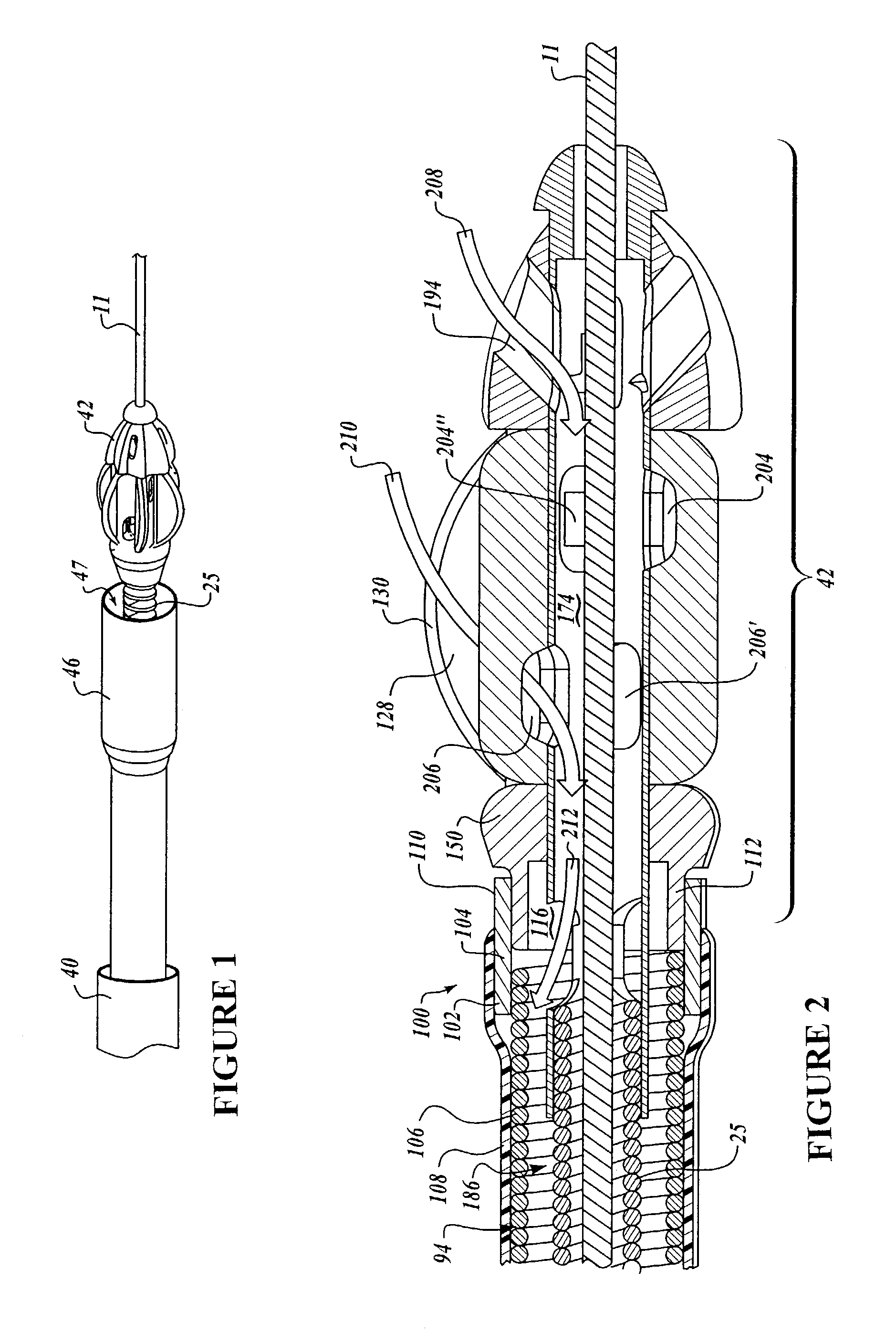
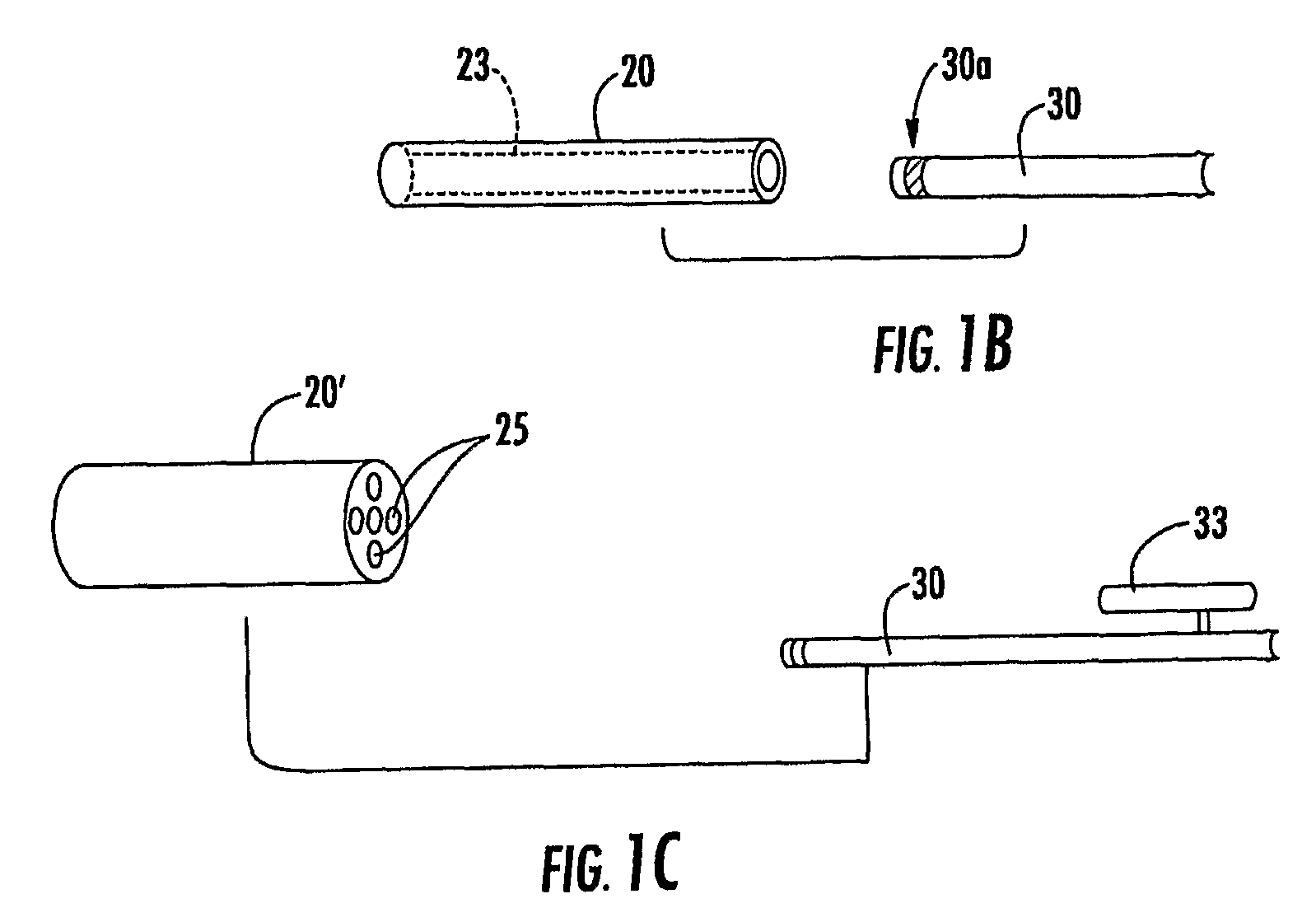
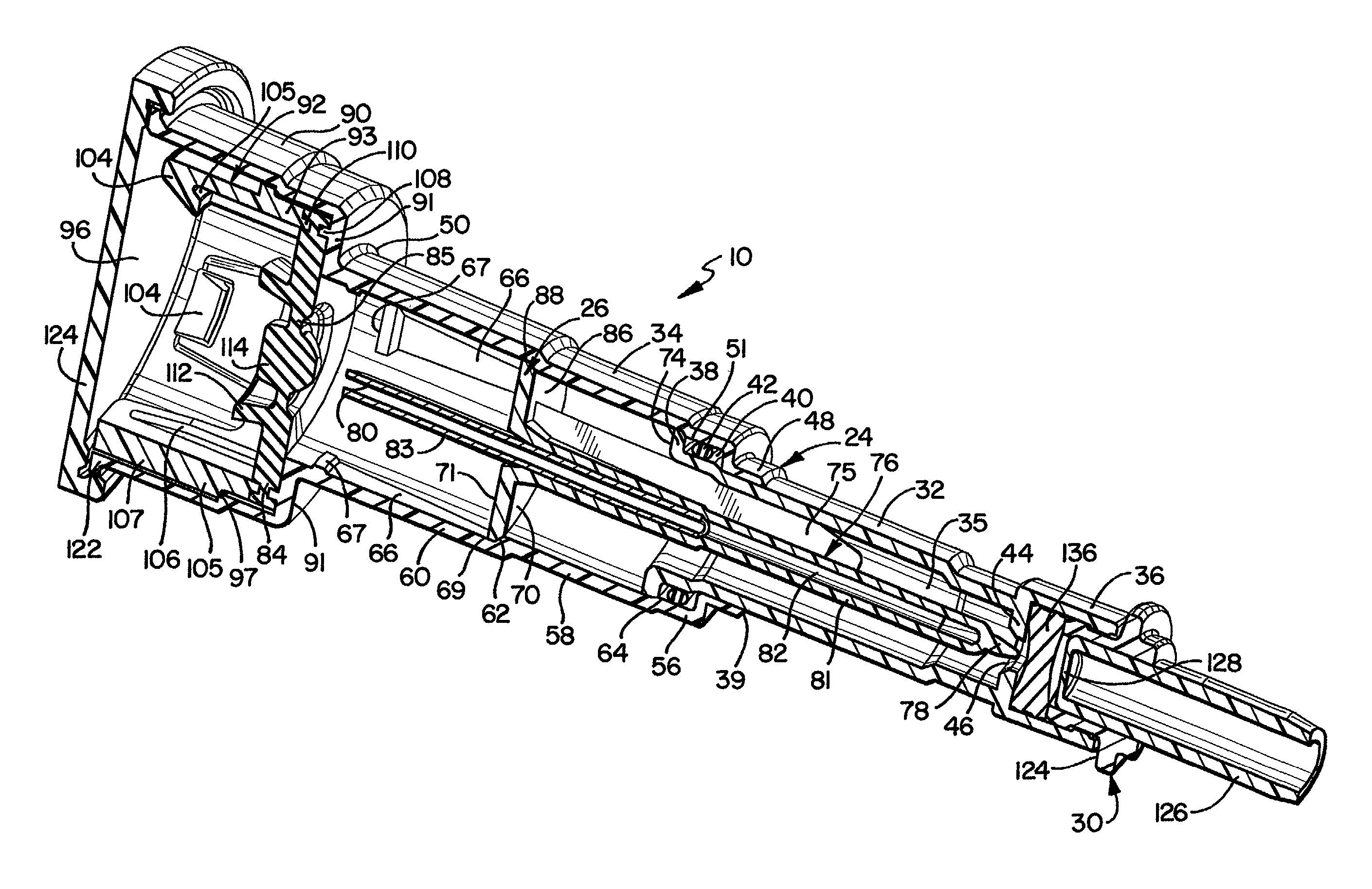
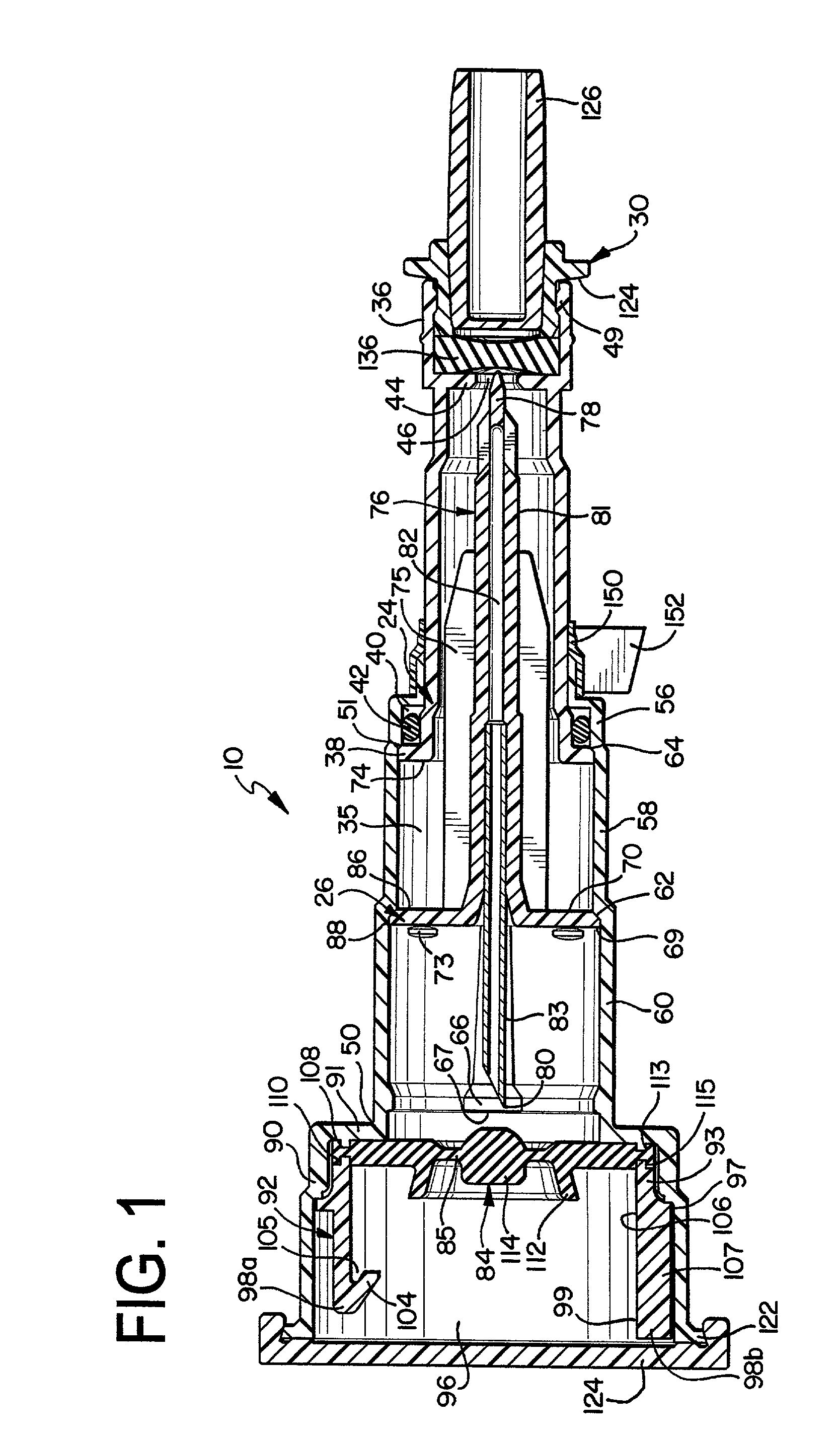
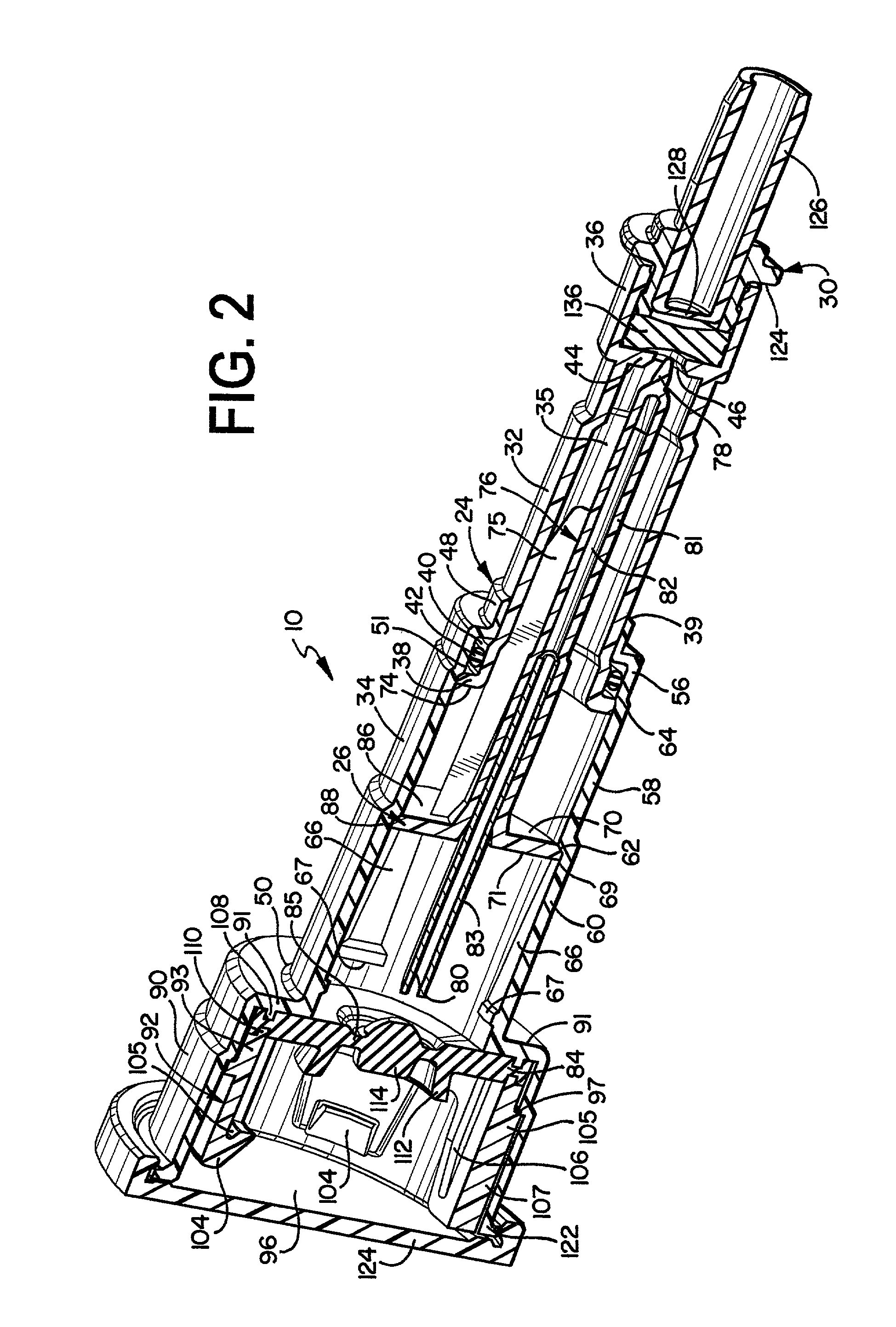
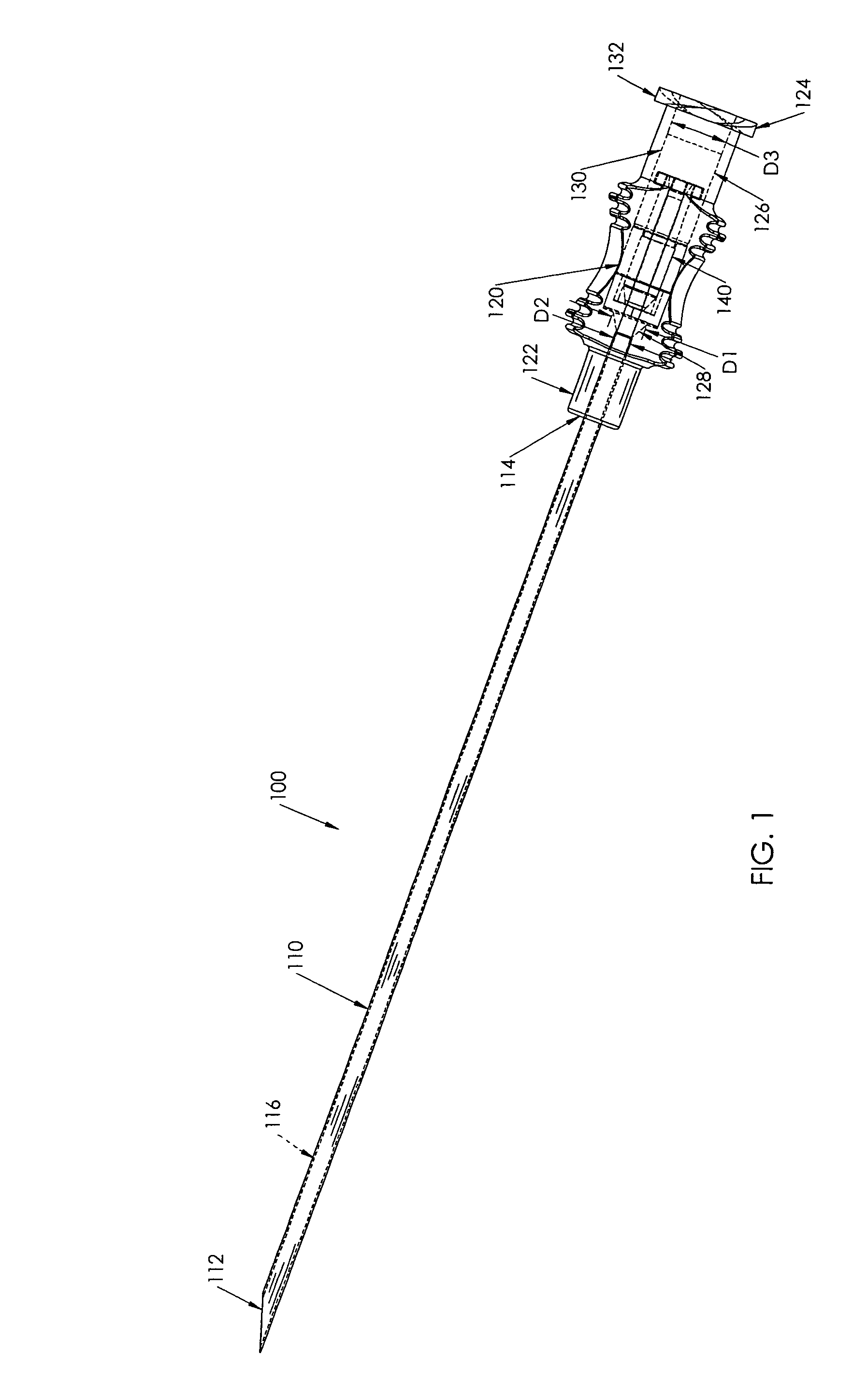
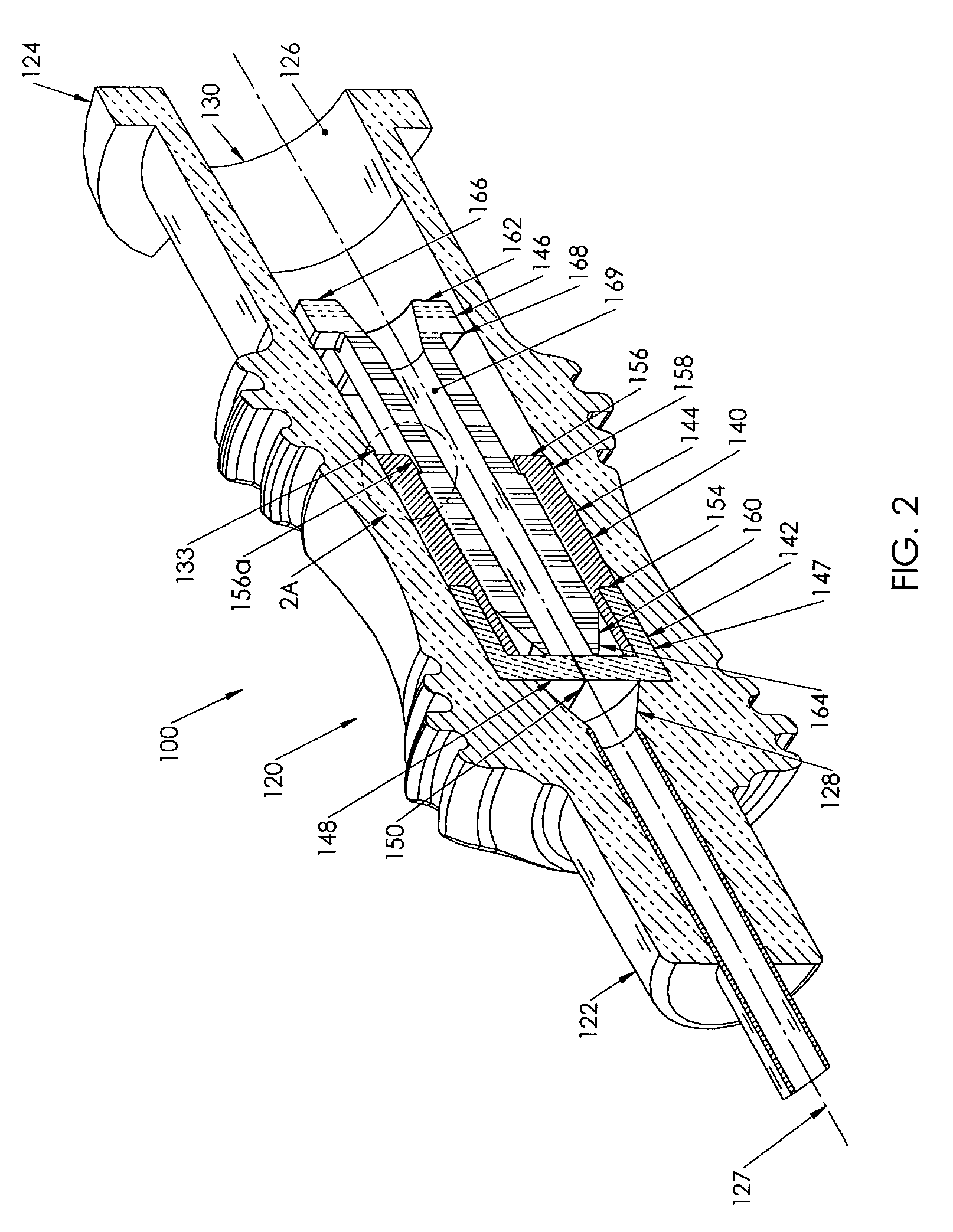
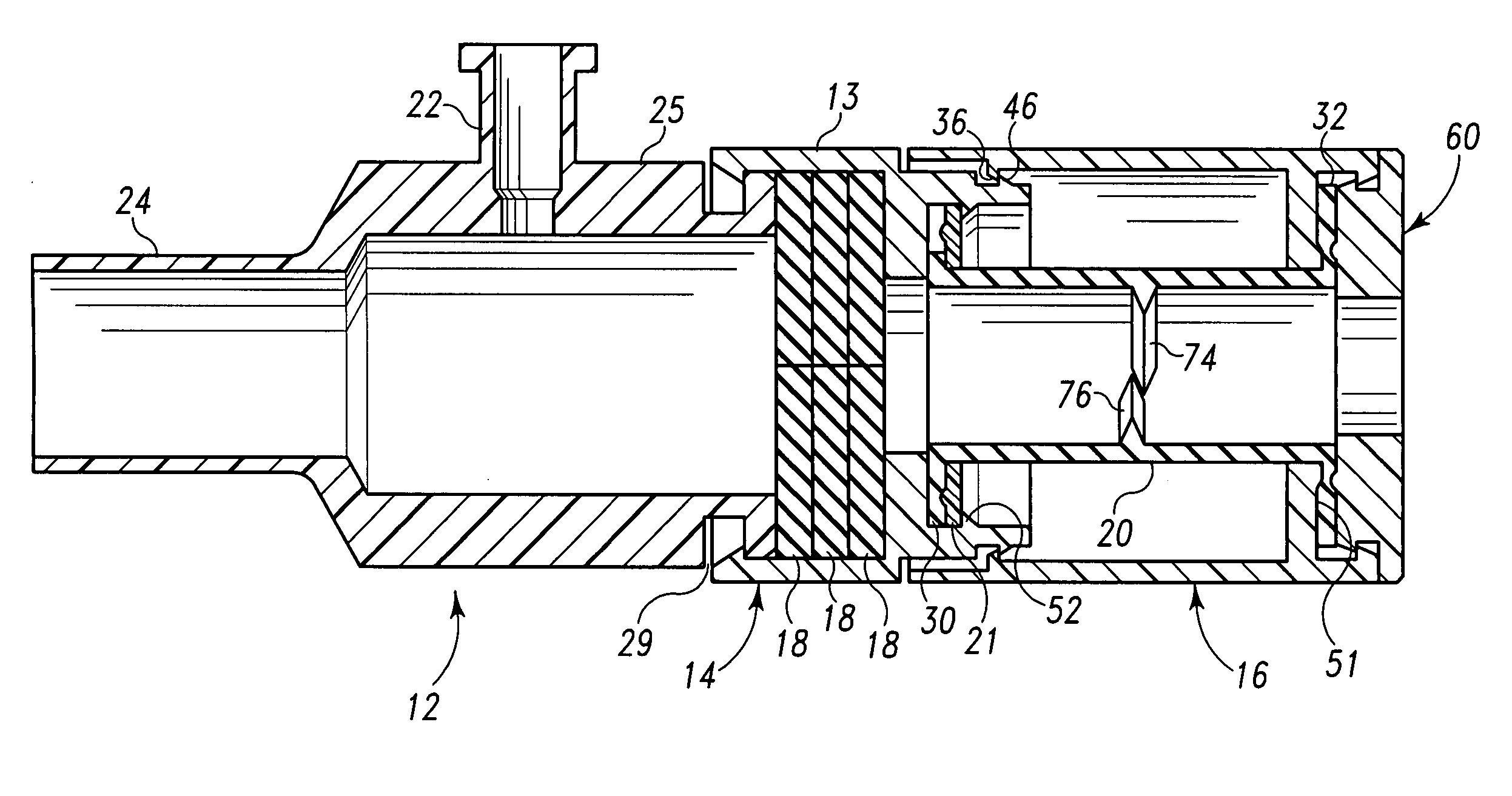
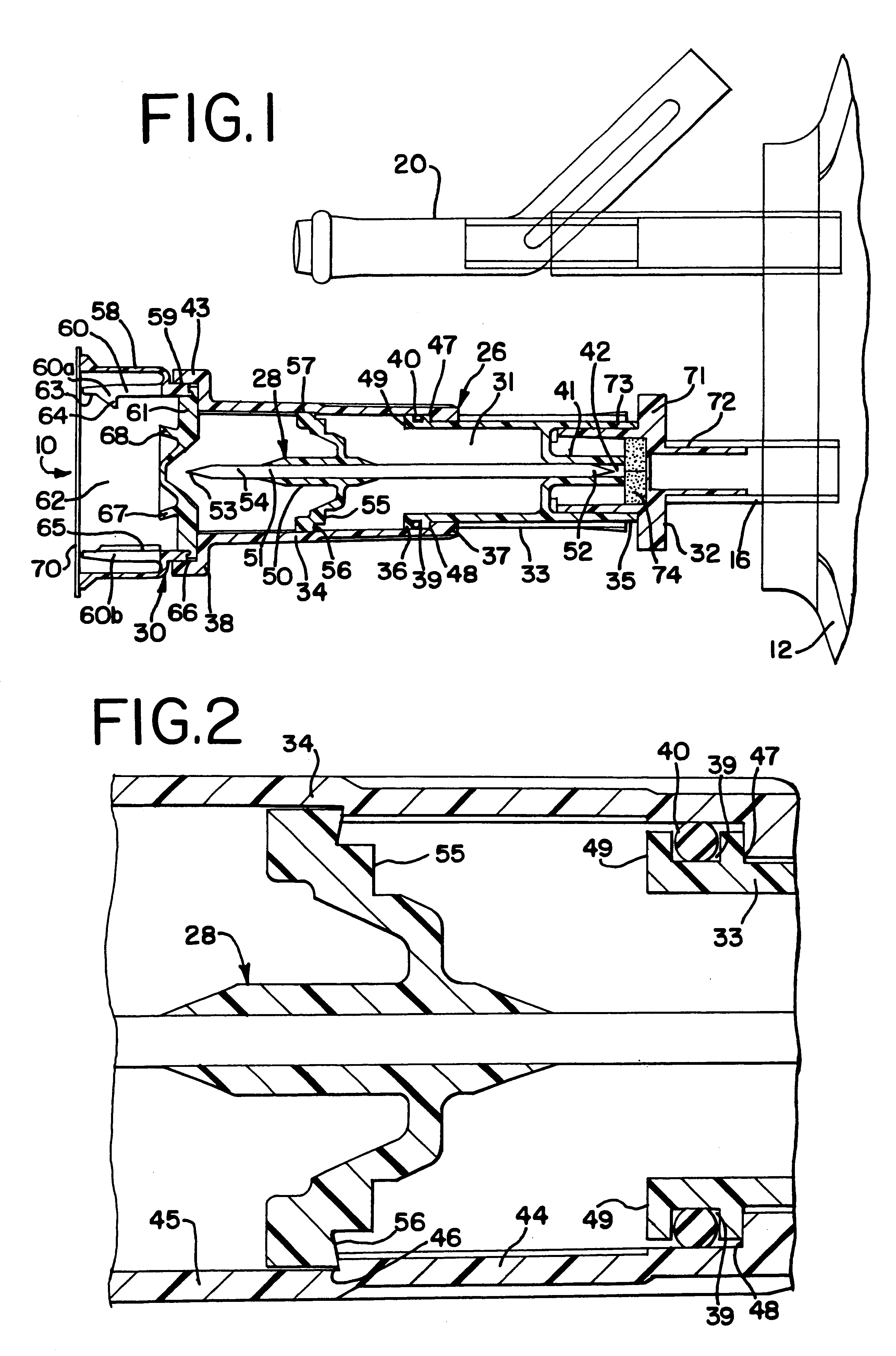
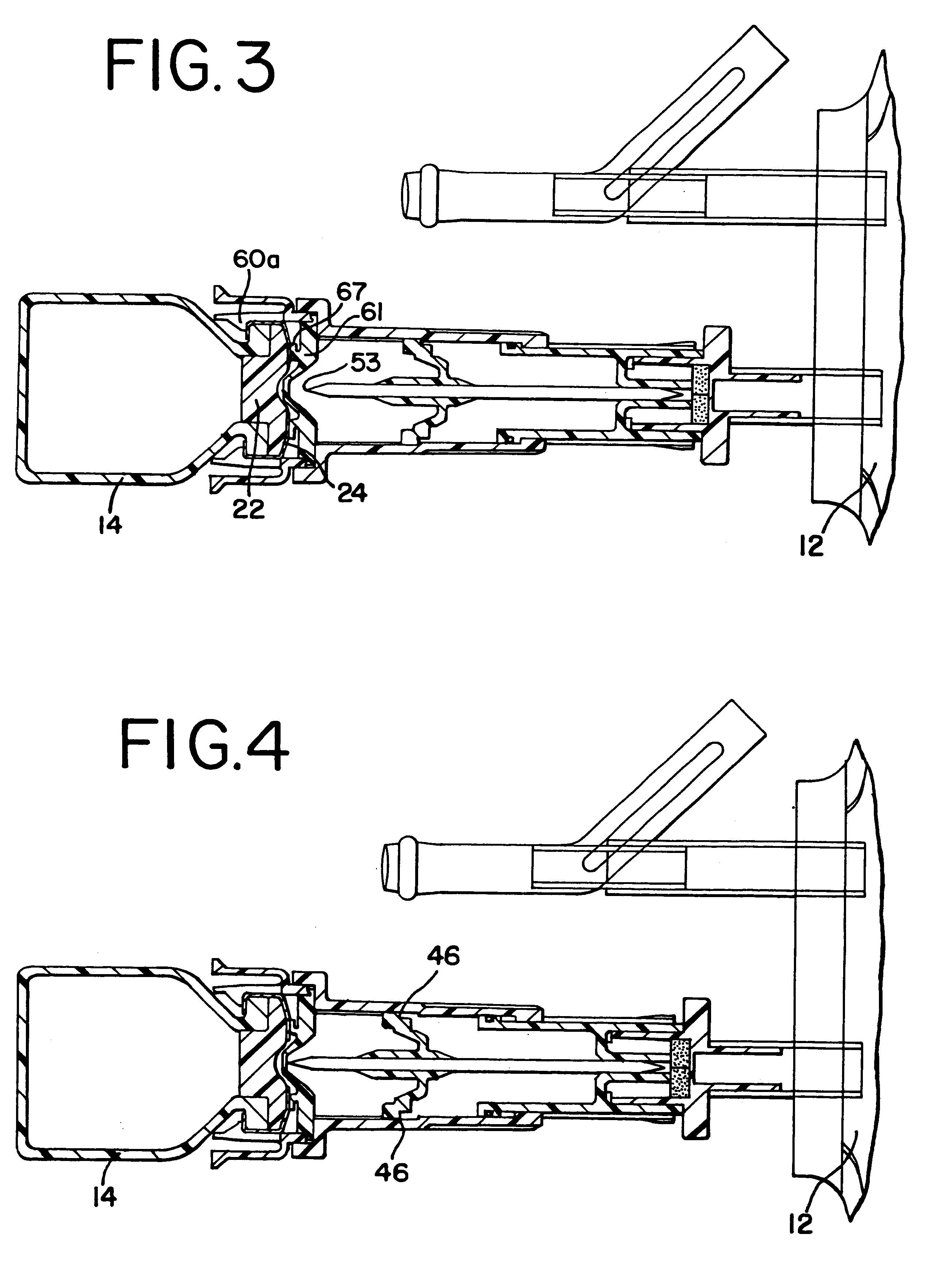
Needle with sealing valve
A needle assembly (100) having a hollow needle (110) with a pointed distal end (112) and a proximal end (114). The assembly also includes a needle hub (120) having a distal hub end (122) fixedly connected to the needle (110), a proximal hub end (124) having an opening, and a passageway (126) extending therethrough between the distal end and the proximal end. A valve (140) is disposed within the passageway (126), wherein the valve comprises a sealing member (142) having at least one through-opening (150) disposed therein and a plunger (146) disposed proximate the sealing member (142) and slidable between a first position wherein the at least one through-opening (150) is closed such that the hollow needle (110) and the proximal hub end (124) are not in fluid communication with each other and a second position wherein the plunger (146) biases the at least one through-opening (150) to an open position, such that the hollow needle and the proximal hub end are in fluid communication with each other. A method of using the needle assembly (100) is also disclosed.
Owner:MEDICAL COMPONENTS INC
Apparatus and methods for clearing obstructions from surgical cutting instruments
InactiveUS20060264995A1Rapidly and efficiently removingSave operating timeSuction irrigation systemsSuction drainage systemsSurgical siteEngineering
The present invention relates to apparatus and methods for facilitating removal of obstructions from a surgical cutting instrument during a surgical procedure. The apparatus of the present invention is configured to interrupt flow in aspiration tubing when obstructions are detected in the cutting instrument. The apparatus then causes compression of the aspiration tubing to flush fluid towards the cutting instrument, thereby unclogging the instrument in a fast and efficient manner without the need to remove the instrument from the surgical site.
Owner:SAPPHIRE MEDICAL
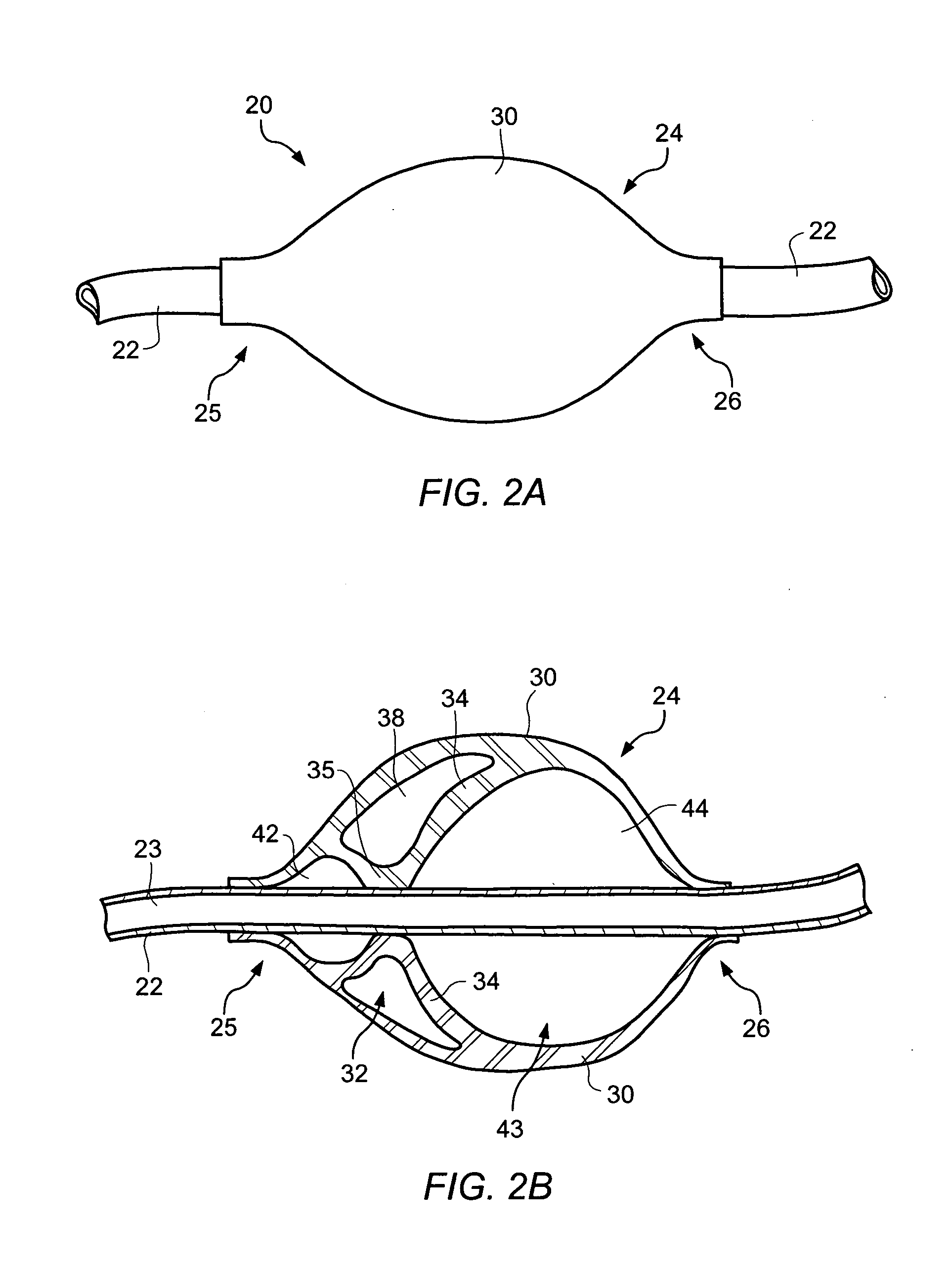
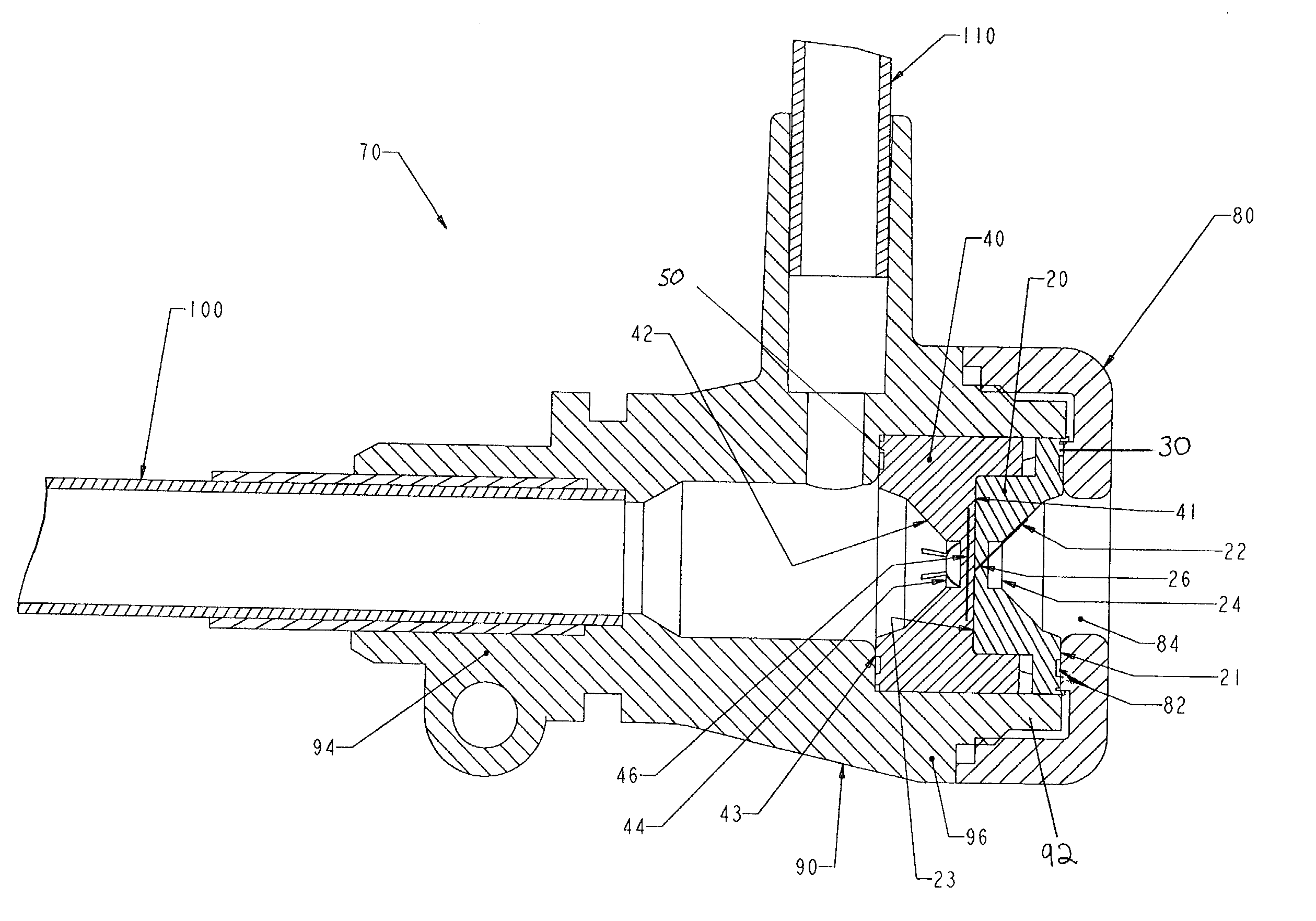
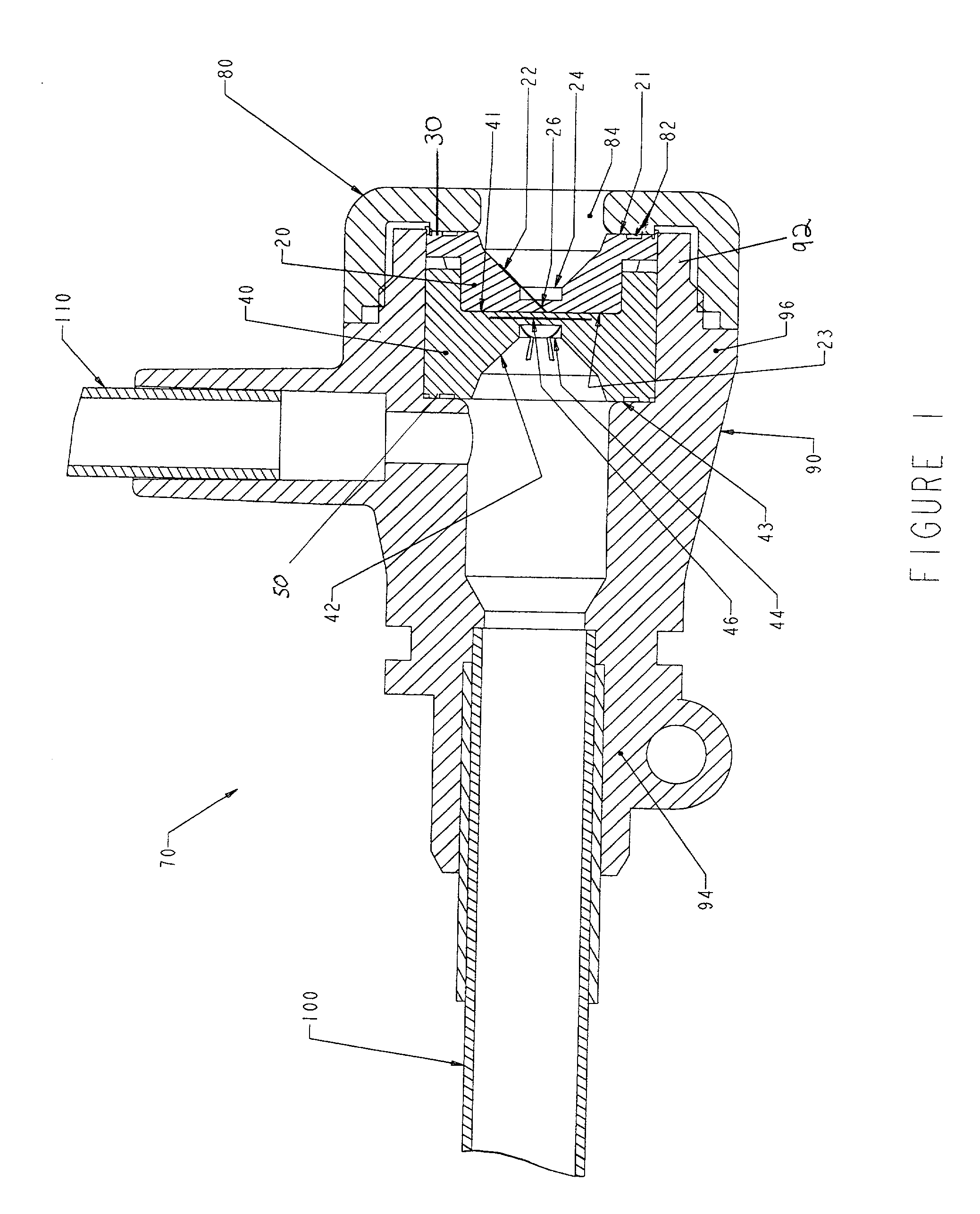
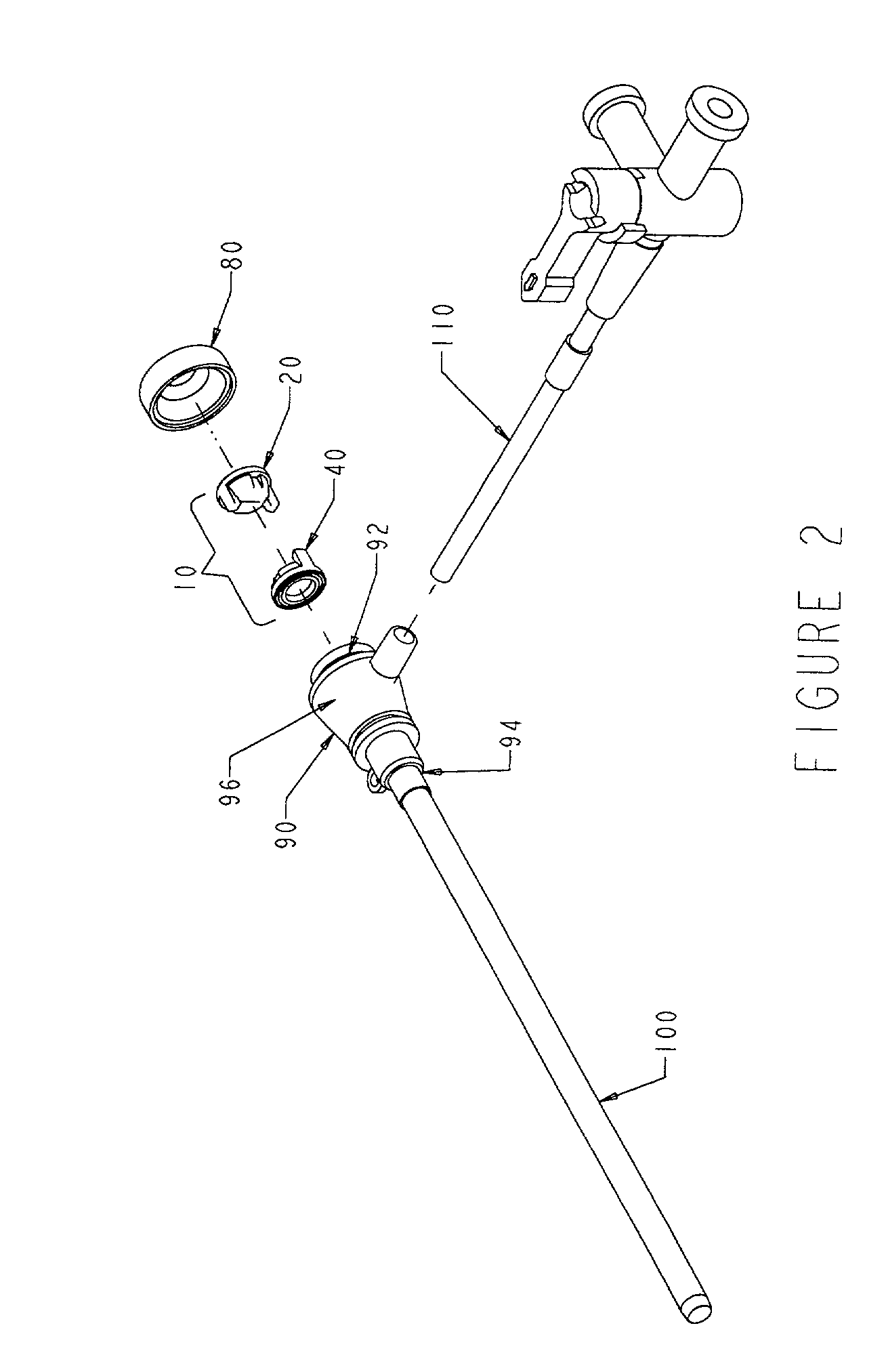
Hemostasis valve
A hemostasis cannula unit including a valve housing, a cap, and a hemostasis valve, wherein the hemostasis valve includes a proximal valve gasket and a distal valve gasket compressed against the valve gasket by the valve housing, wherein the proximal valve gasket is the same shape as the distal valve gasket.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Hemostasis valve
A hemostasis cannula unit including a valve housing, a cap, and a hemostasis valve, wherein the hemostasis valve includes a valve gasket and a valve membrane compressed against the valve gasket by the valve housing, wherein the valve gasket is thicker than the valve membrane.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
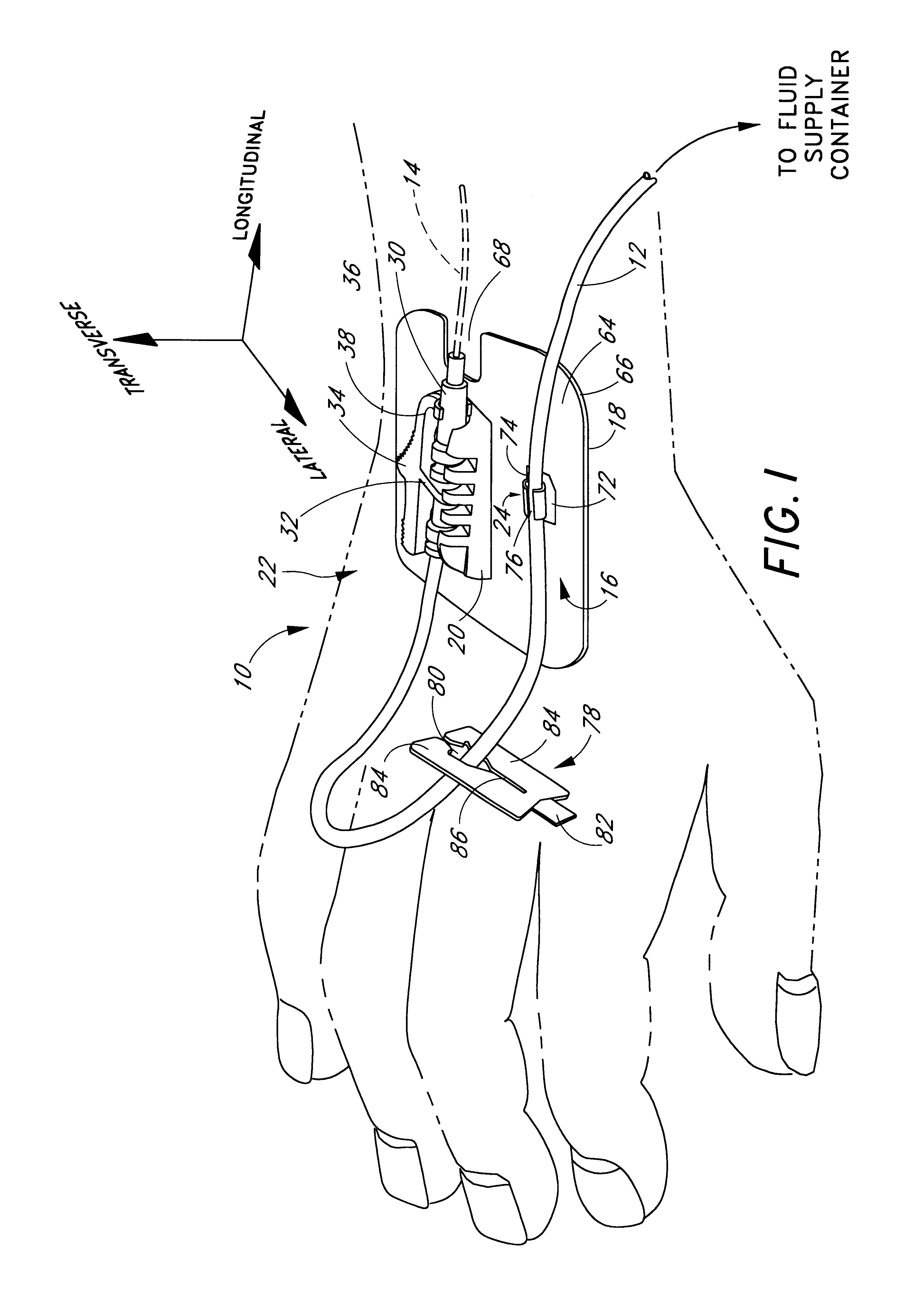
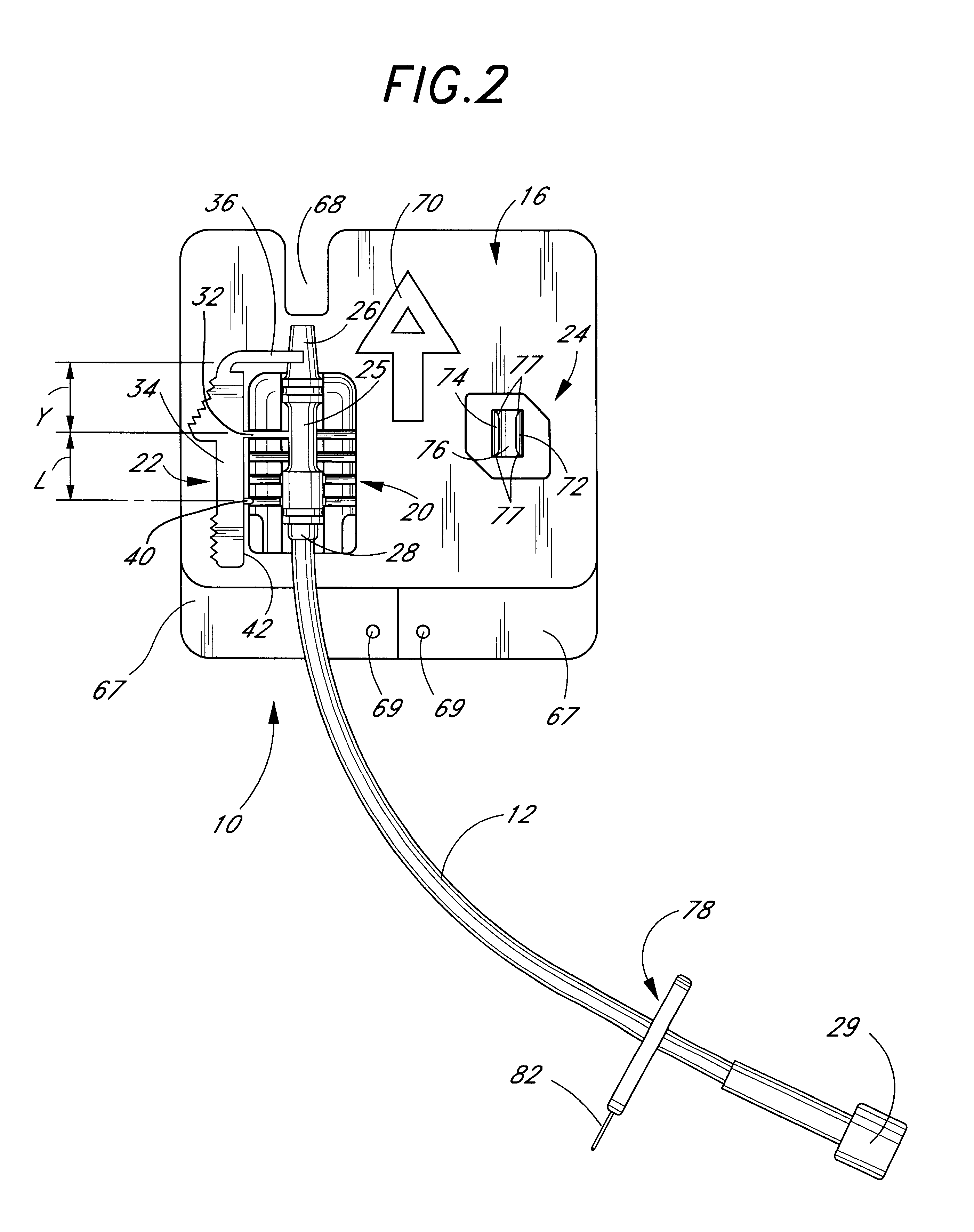
Catheter anchoring system
InactiveUS6837875B1Prevent slidingPrevent longitudinal movementSurgeryCatheterCATHETER ADAPTERInterconnection
A catheter anchoring system is provided to securely anchor to a patient's skin a catheter and fluid supply tube interconnection. The anchoring system comprises a retainer configured to receive a catheter adaptor in a variety of positions. The adaptor interconnects the catheter and the fluid supply tube. In one embodiment the adaptor has a radial recess that circumscribes the adaptor. The anchoring system additionally includes a flexible, adhesive anchor pad which can supports a tube clip, as well as the retainer. The retainer includes a channel that is configured to receive the adaptor in a snap-fit manner. The retainer also includes a plurality of laterally arranged projections that extend from a channel wall and into a channel. Each radial recess is sized to receive and to capture at least a portion of the projection of the retainer so as to inhibit the adaptor from moving within the channel.
Owner:VENETEC INT INC
Surgical instrument seal assembly and triple lead thread
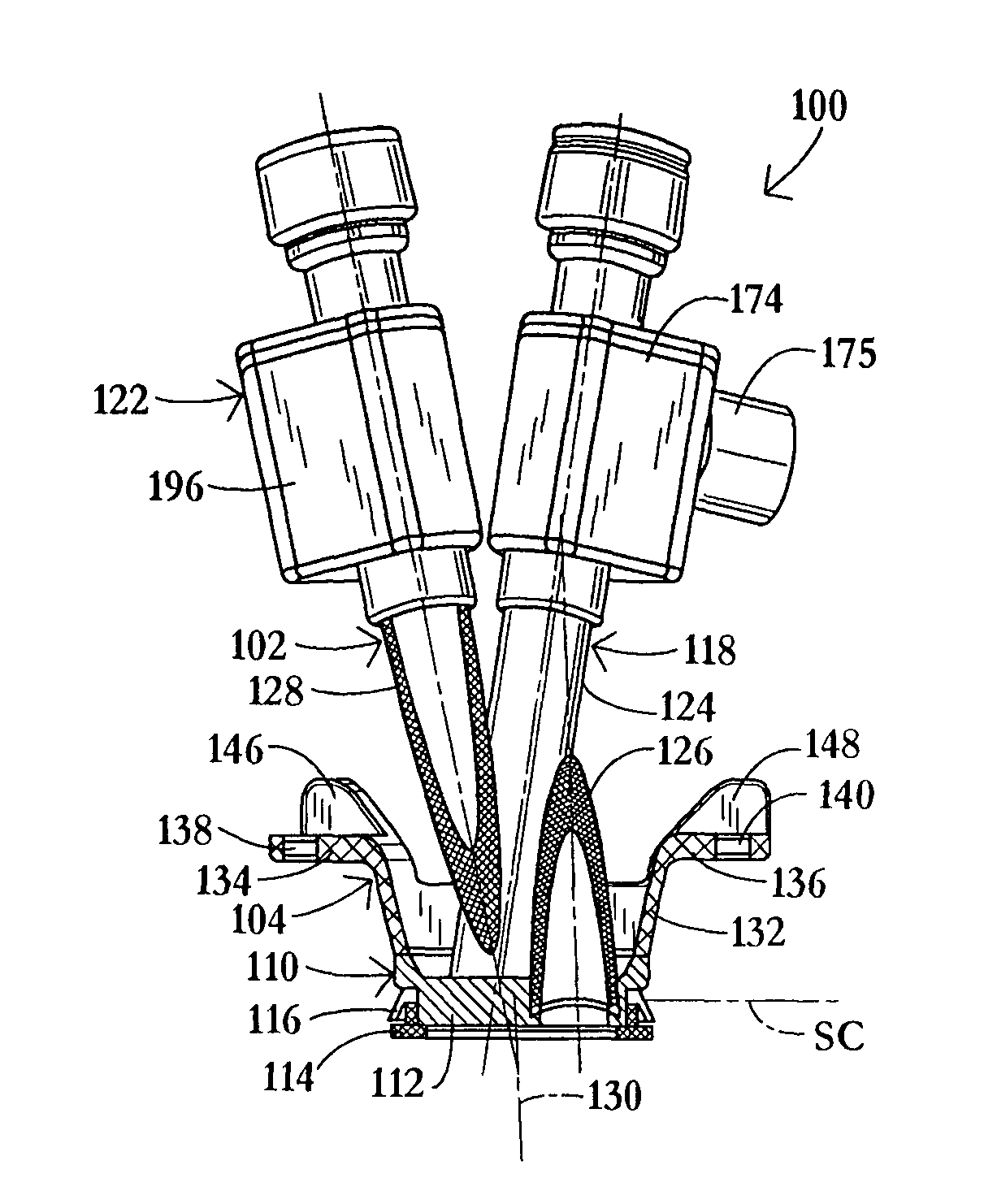
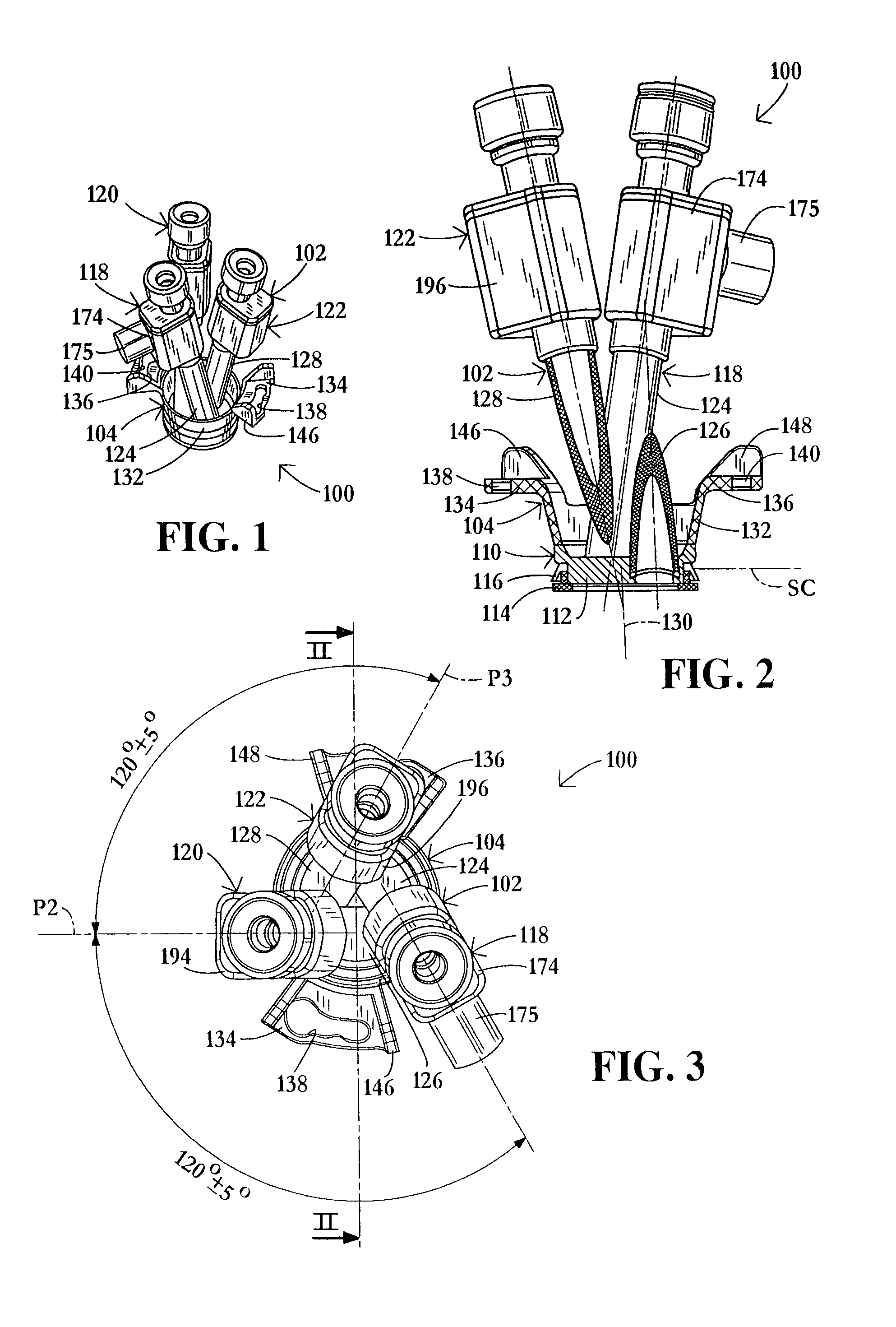
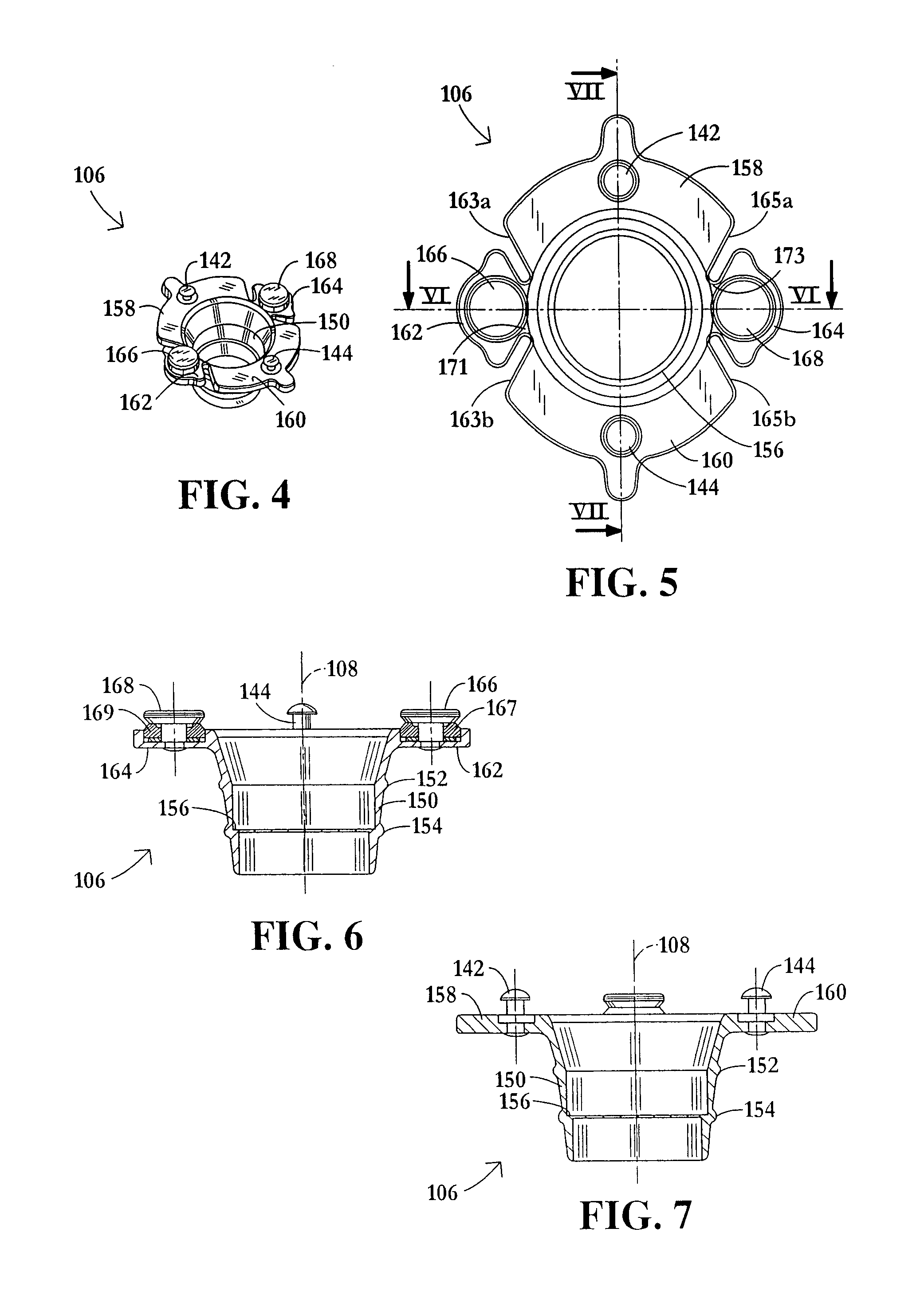
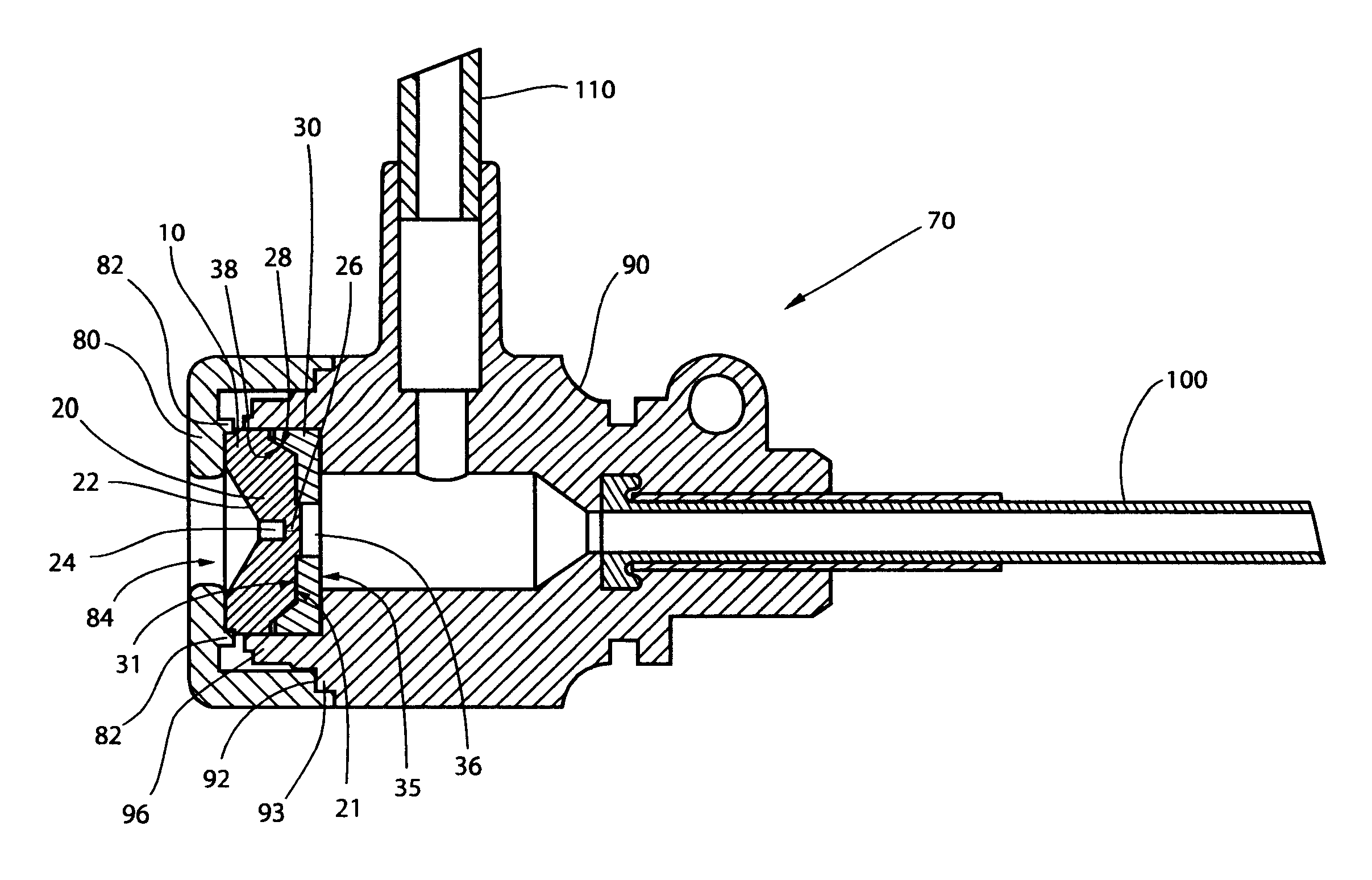
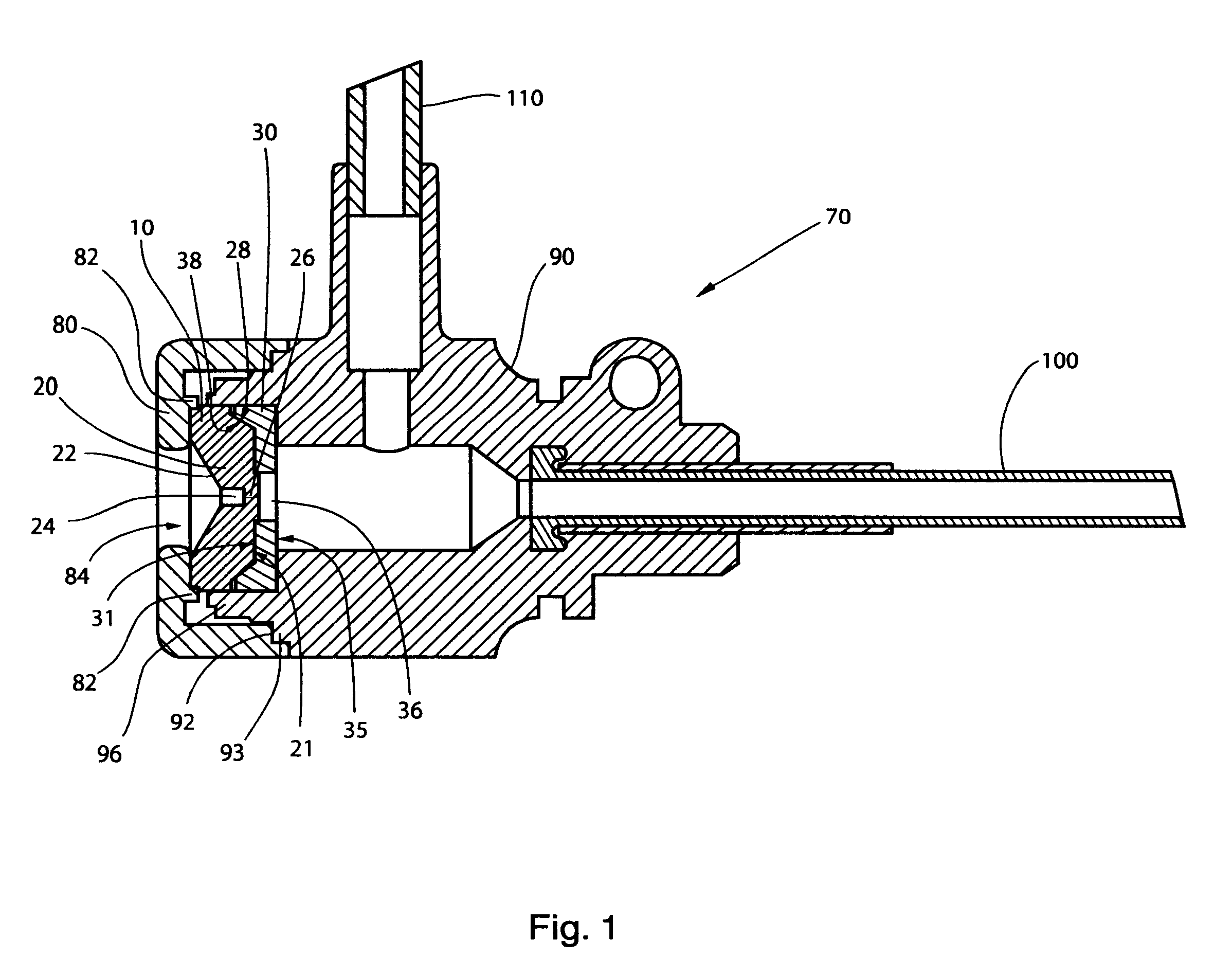
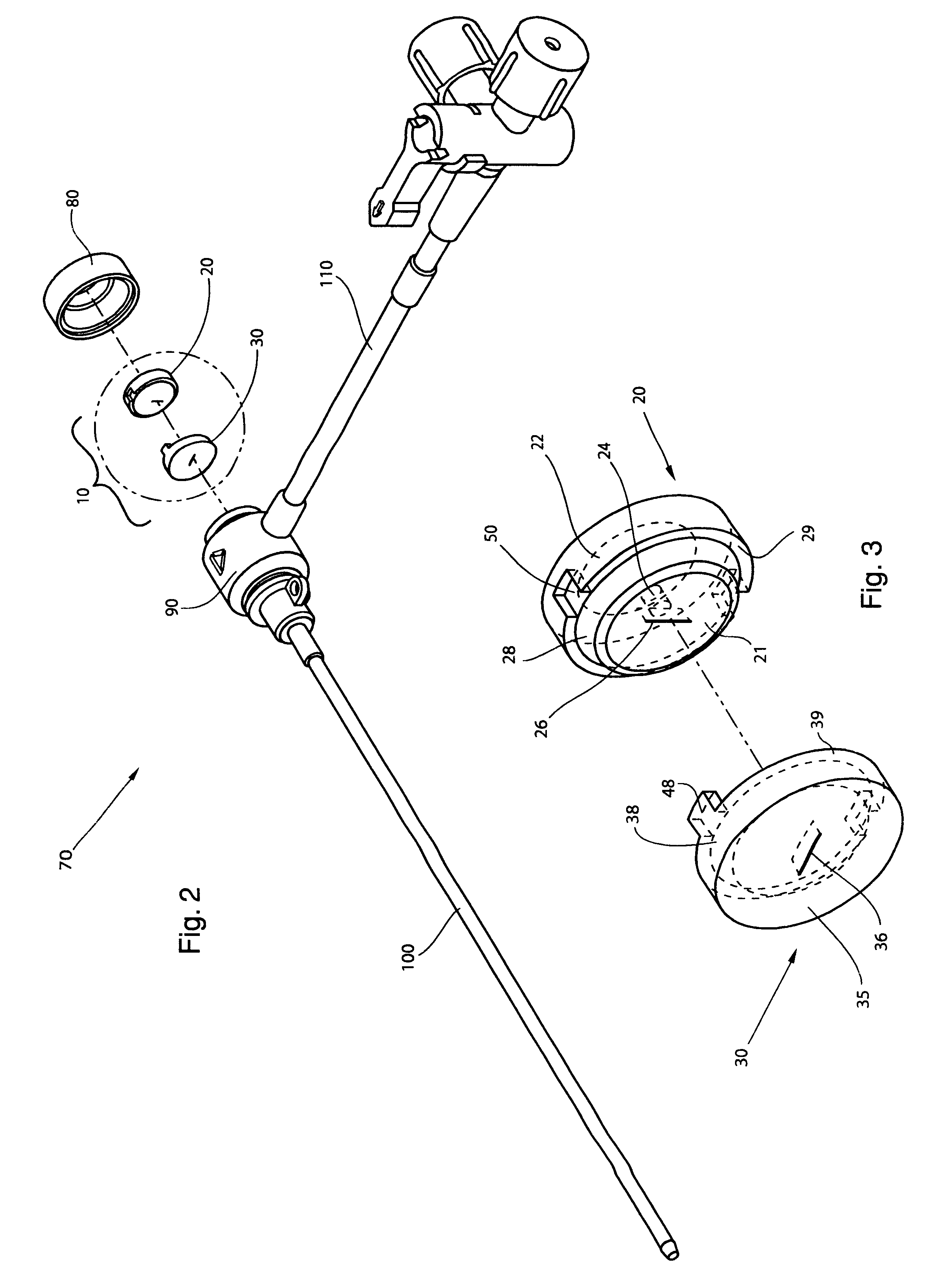
InactiveUS20060217665A1Facilitate pivotal movementEasy to moveCannulasInfusion syringesEngineeringHourglass
A surgical instrument has an hourglass instrument seal operably coupled to the interior of the valve seal assembly. The hourglass instrument seal includes a top flange, a free floating lower flange and a rippled junction adjoining a top conical portion and bottom conical portion. An anti-inversion assembly biases the top flange apart from the lower flange. A tilt subassembly enables pivotal movement of the seal assembly using a ball and socket. Additionally, the cap housing may include a ball socket for slidably engaging the lower spherical section of the tilt assembly. A duckbill valve includes a pair of flaps, each having a plurality of reinforcing ribs. A fluid port is disposed at an acute upward angle relative to the channel. A cannula tube includes a plurality (e.g., three) independent parallel sets of evenly spaced threads.
Owner:LAPAROSCOPIC PARTNERS
Injection port valve
Injection port valves are generally discussed herein and more particularly to port valves that have a valve housing with a nozzle having an inlet opening, a body section, a skirt section, an interior cavity, and a valve seat at an intersection of the first nozzle and the body section in the interior cavity. A piston may be positioned within the interior cavity of the valve housing having a body section having an exterior surface, an upper piston section, a seal surface, and a piston cavity, and wherein the body section includes a wall layer that emits lubricant from within the wall layer to the exterior surface. A nut, which may be a Luer nut, having a flow passage in communication with a discharge nozzle having a discharge lumen may be attached to an end of the skirt section of the valve housing to thereafter connect to an infusion line. A spring can be positioned within the piston cavity and abutting against the nut, wherein when used, the spring being in a first position when the seal surface of the piston is abutted against the valve seat of the valve housing, and the spring being in a second position when the seal surface of the piston is separated from the valve seat.
Owner:B BRAUN MEDICAL
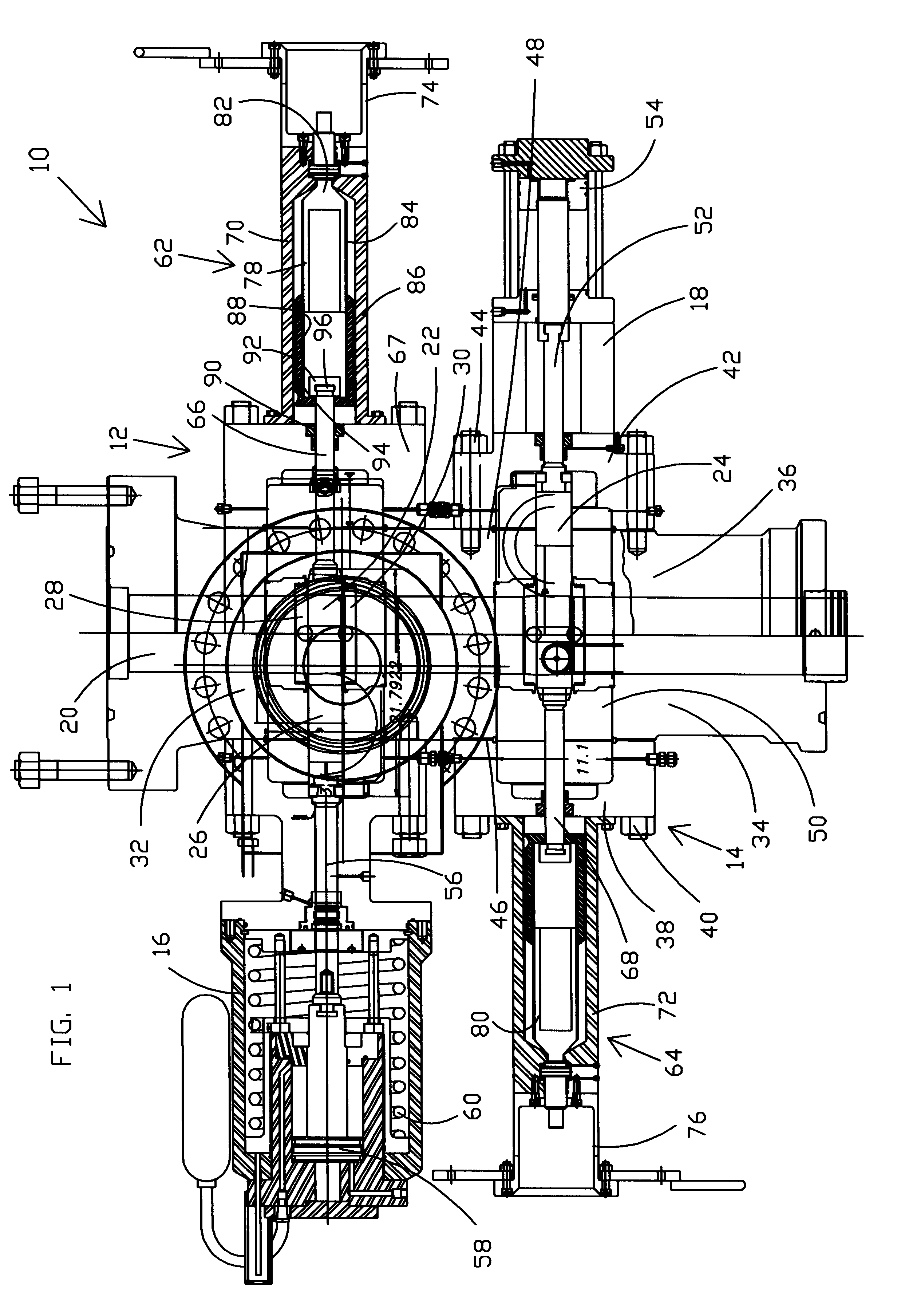
Method and apparatus for replacing BOP with gate valve
Owner:WORLDWIDE OILFIELD MACHINE INC
Hemostatic valve assembly
An iris-type valve assembly for controlling a flow of fluid. The assembly comprises a base member, a rotatable member, and an elongated elastomeric valve sheath. The distal end of the valve sheath is secured to the base member and the proximal end of the valve sheath is secured to the rotatable member. At least one of the valve sheath ends includes a flange that is secured to a valve-receiving surface of the base member or the rotatable member. The base member and the rotatable member are aligned to define an elongated passageway therethrough for passage of an interventional device, and the elastomeric valve sheath is disposed along the passageway and has a longitudinal opening therethrough for passage of the interventional device. Upon rotation of the rotatable member relative to the base member the longitudinal opening of the valve sheath is selectively constrictable to comprise a seal around the interventional device. The assembly may be provided with ratcheting members to inhibit recoil of the rotatable member, and rib members along the inner surface of the valve sheath to enhance the seal provided by the assembly.
Owner:COOK MEDICAL TECH LLC
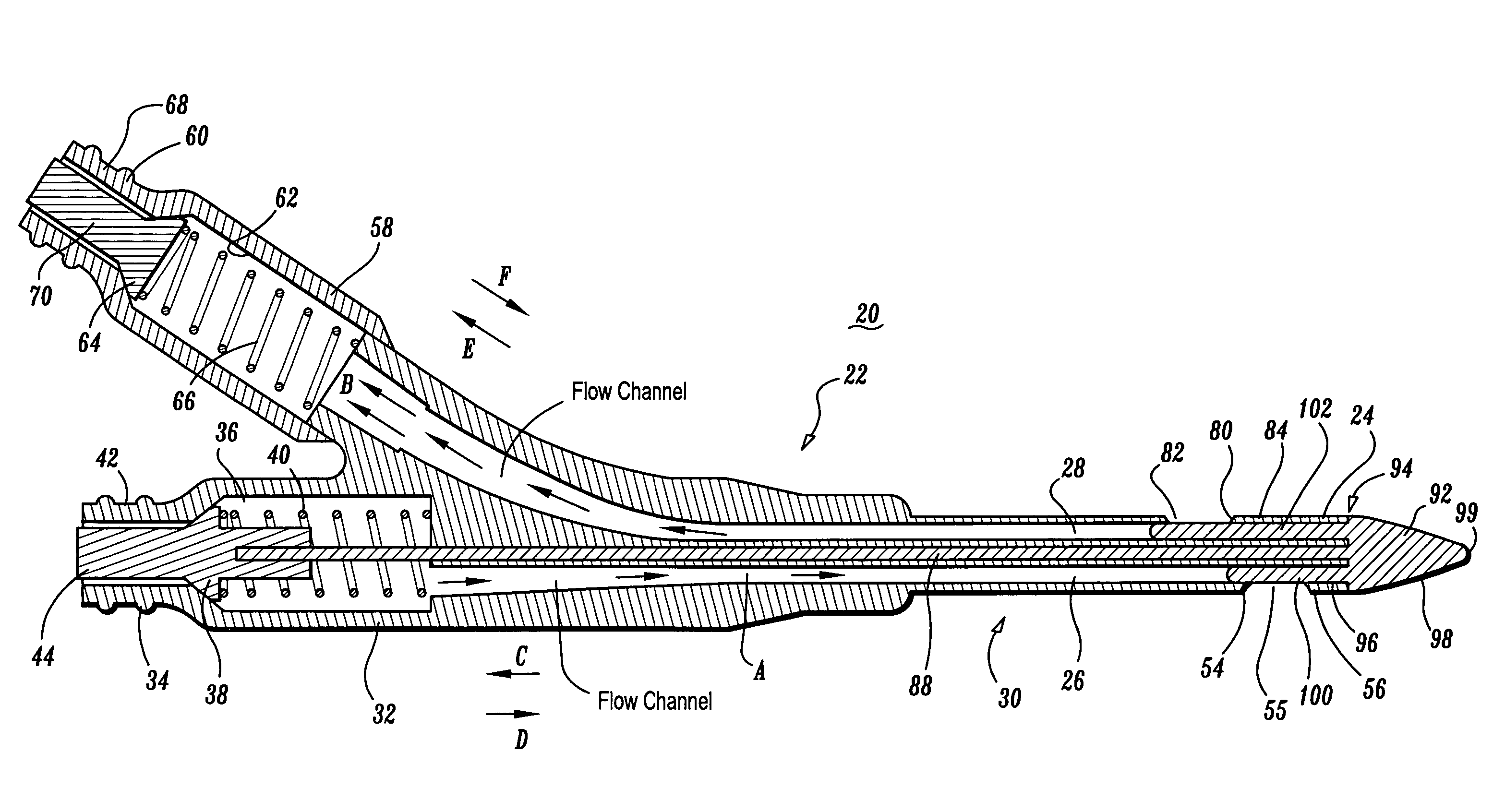
Valved catheter
ActiveUS7211074B2Facilitates bi-directional fluid flowPrevents evacuation and anti-coagulant leakageMulti-lumen catheterIntravenous devicesVALVE PORTCatheter device
A catheter apparatus is provided that includes a tubular body having a distal end. The body defines first and second lumens. The first lumen has a first adapter that includes a first valve biased to seal the proximal end. The first lumen defines a first lateral port and the second lumen defines a second lateral port. A push rod is connected to the first valve for corresponding movement therewith and extends to a tip. The tip includes a first member extending into the first lumen and a second member extending into the second lumen such that the first member seals the first lateral port and the second member seals the second lateral port in a closed position of the tip. The first valve is engageable such that fluid communication is established between the proximal end of the first lumen and the first lumen, and the first and second members move to an open position whereby fluid communication is established. The first and second lumens may be coaxial whereby the first lumen has a first port that seals a second port of the second lumen.
Owner:COVIDIEN AG
Vial connecting device for a sliding reconstitution device for a diluent container
A connector device is disclosed for establishing fluid communication between a diluent container having sidewalls and a drug vial. The connector has a piercing member having a first end and a second end and a central fluid pathway. The piercing member is mounted to the liquid container and has fluid accessing portions hermetically sealed from an outside environment. A vial receiving chamber is associated with the piercing member and is dimensioned to connect to the vial. The vial may be selectively attached to the device without piercing the closure of the vial and without breaching the hermetic seal of the fluid accessing portions of the piercing member. Means are provided for connecting the vial receiving chamber to the liquid container. The device is movable from an inactivated position, where the piercing member is outside the sidewalls and no fluid flows between the liquid container and the drug vial, to an activated position, where fluid flows through the fluid pathway between the liquid container and the drug vial. The device is movable from the inactivated position to the activated position by a force applied to the device outside the liquid container.
Owner:BAXTER INT INC
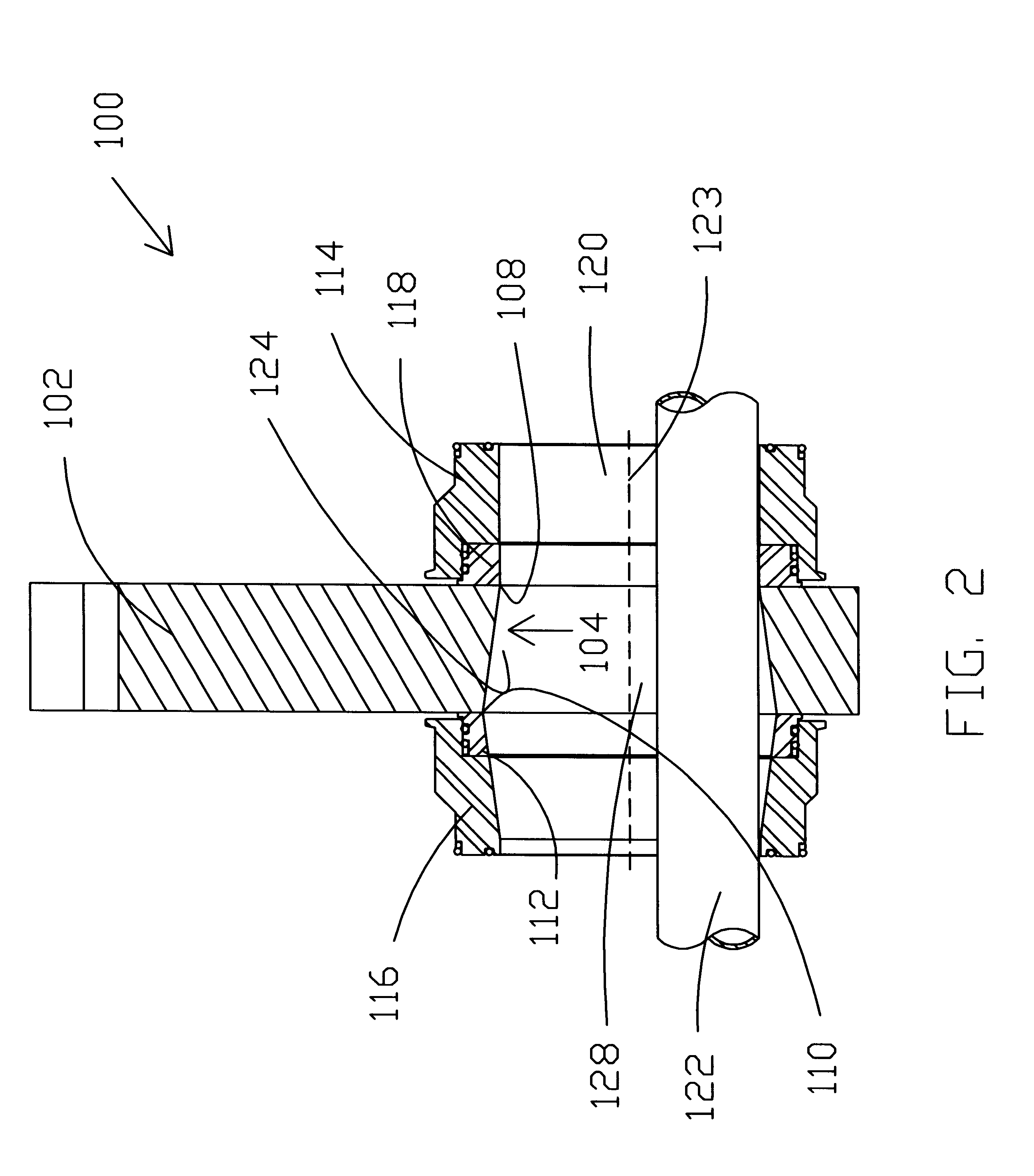
Pressure dome for connecting a transducer with a sealed fluid system
InactiveUS6725726B1Improve compromiseCatheterFluid pressure measurement by mechanical elementsInlet channelTransducer
The invention relates to a pressure dome for connecting a transducer with a fluid system comprising an inlet channel and an outlet flow as well as a measuring chamber in which a fluid is able to circulate and which has a measuring membrane. To improve fluid irculation and simplify handling, the measuring chamber ceiling is configured in the shape of a calotte and the pressure dome is removably coupled to the transducer housing by means of a snap connection.
Owner:MANFRED ADOLFS +1
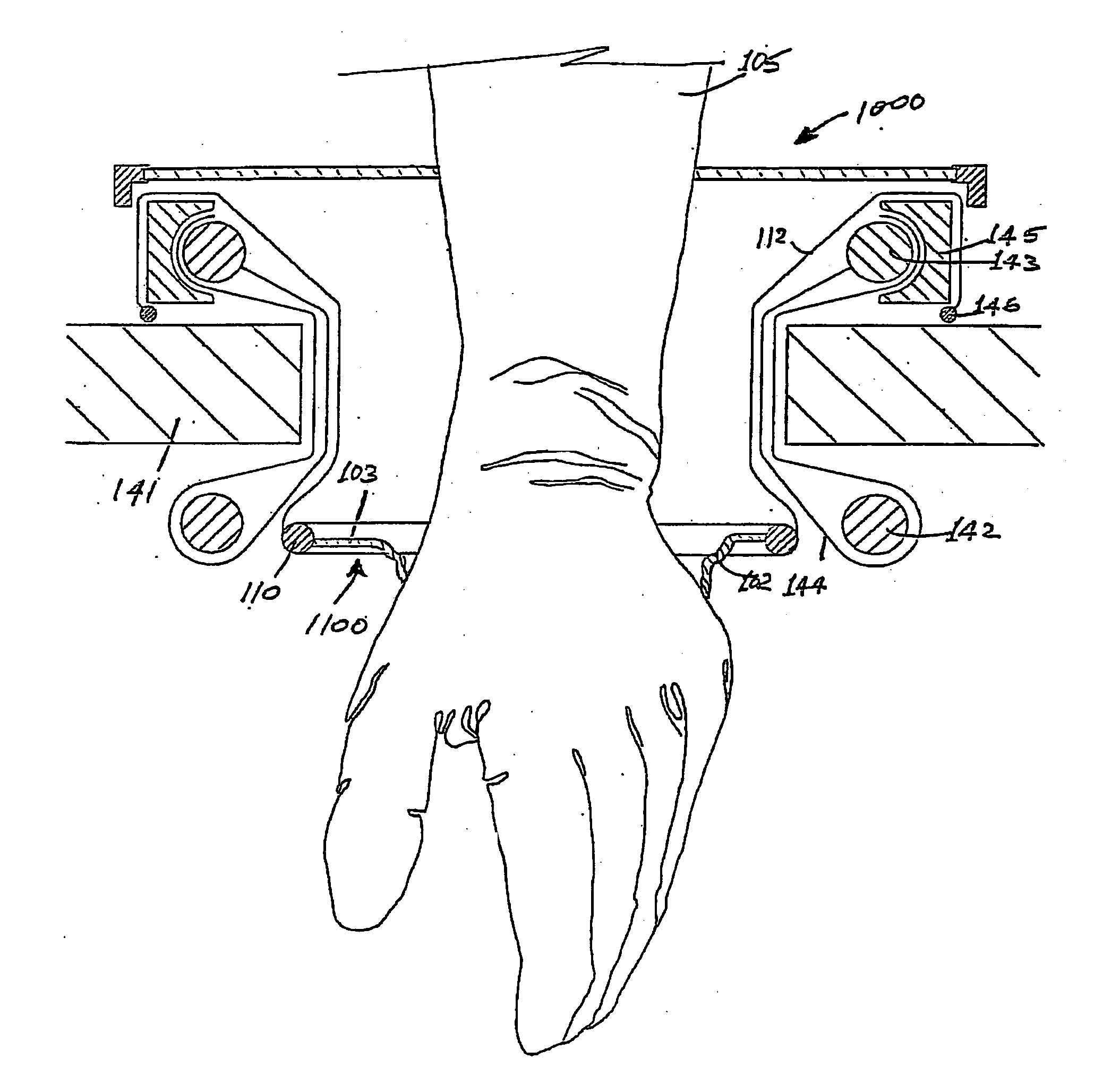
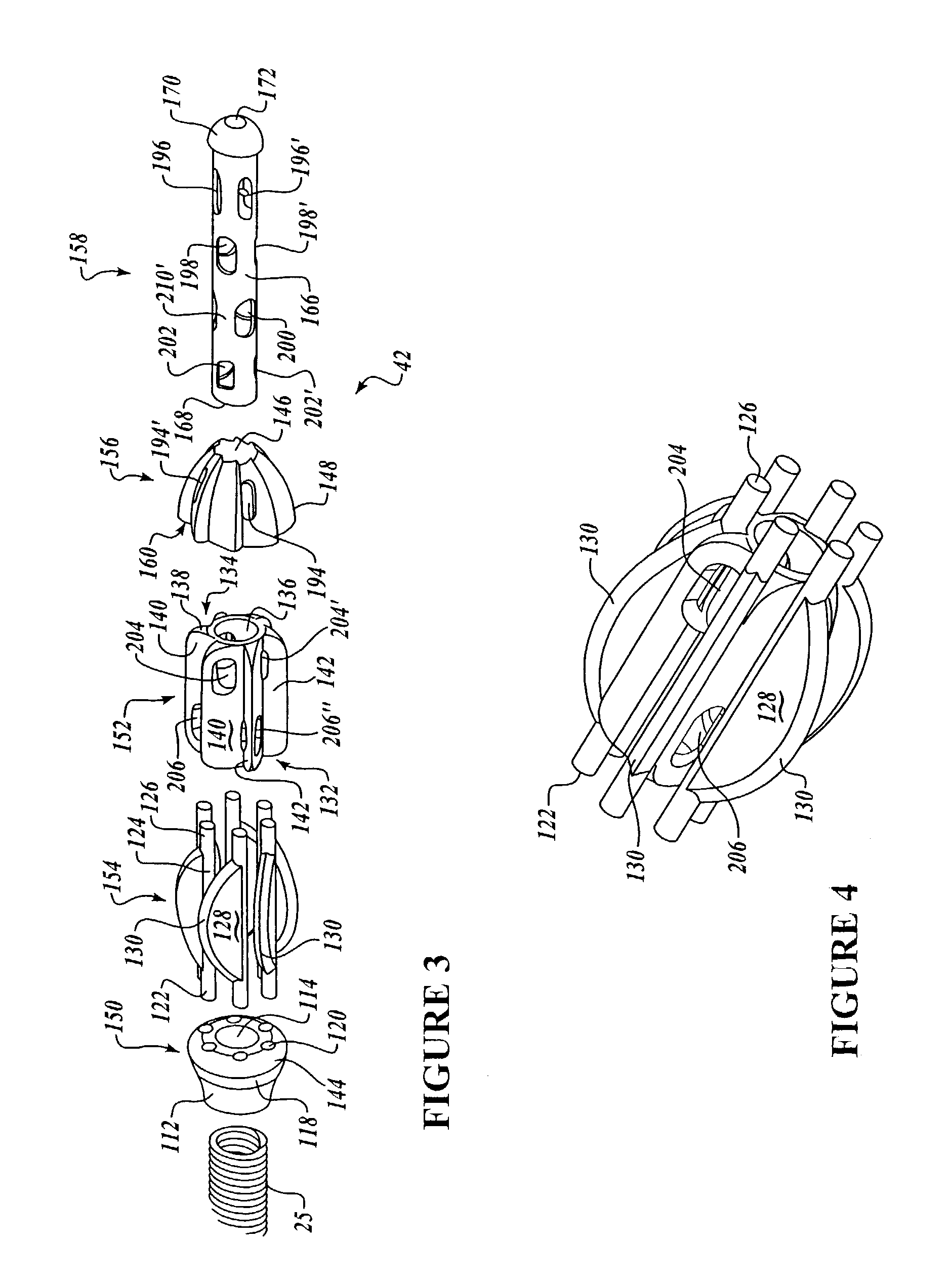
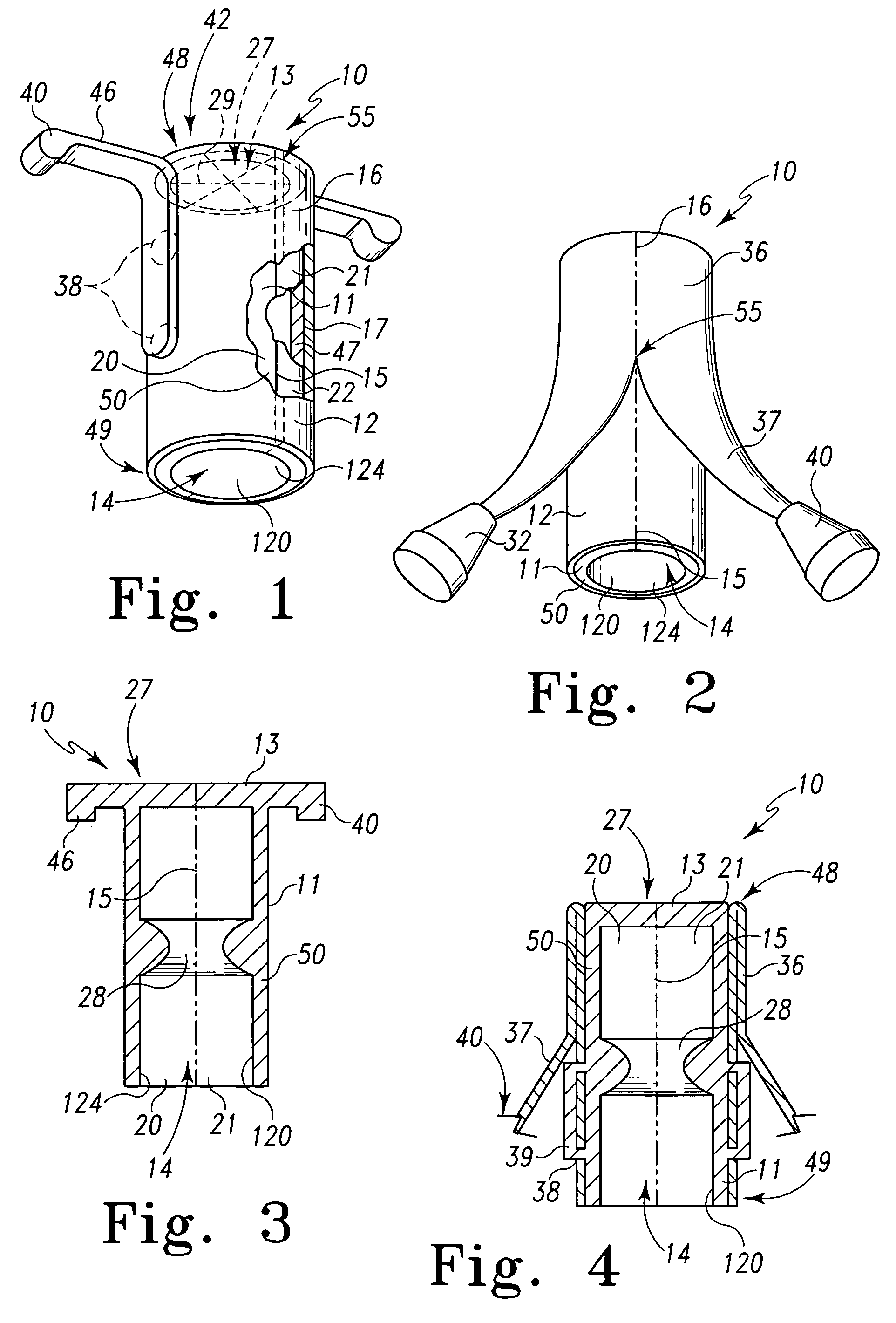
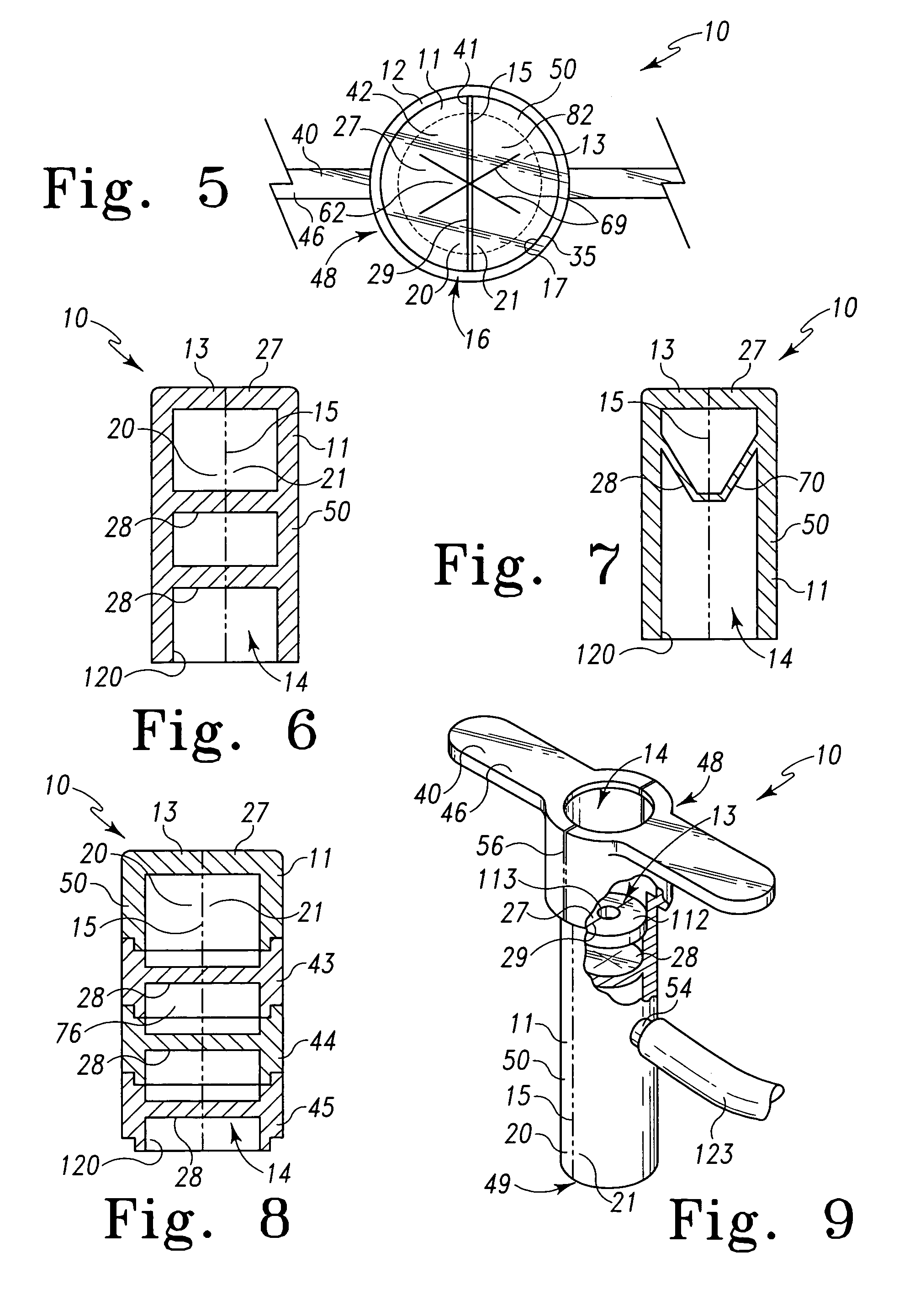
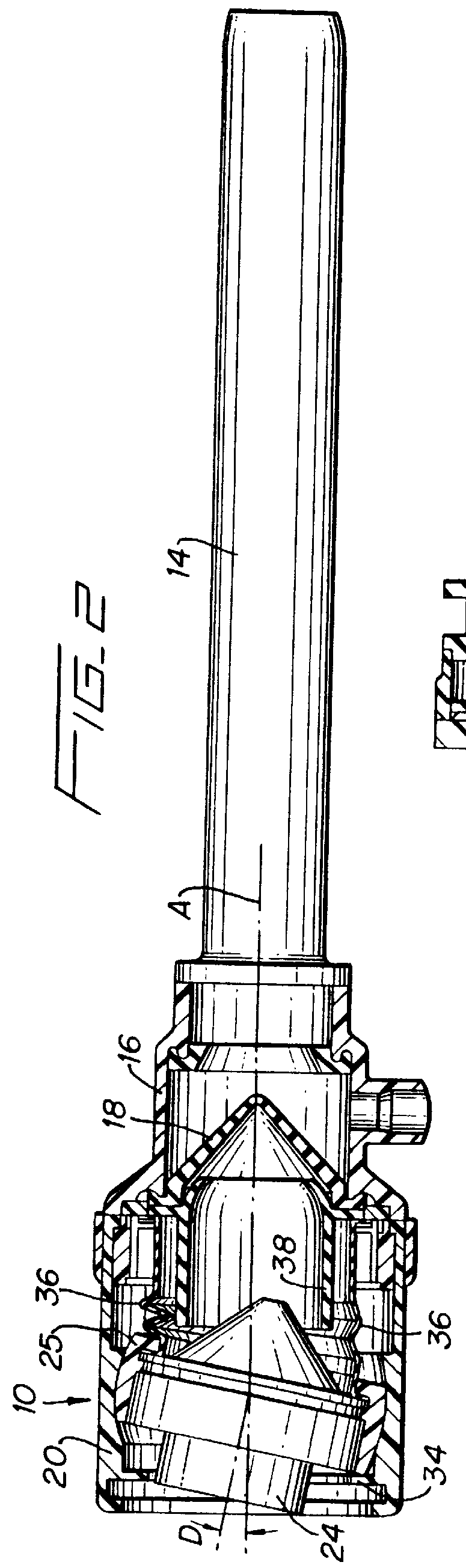
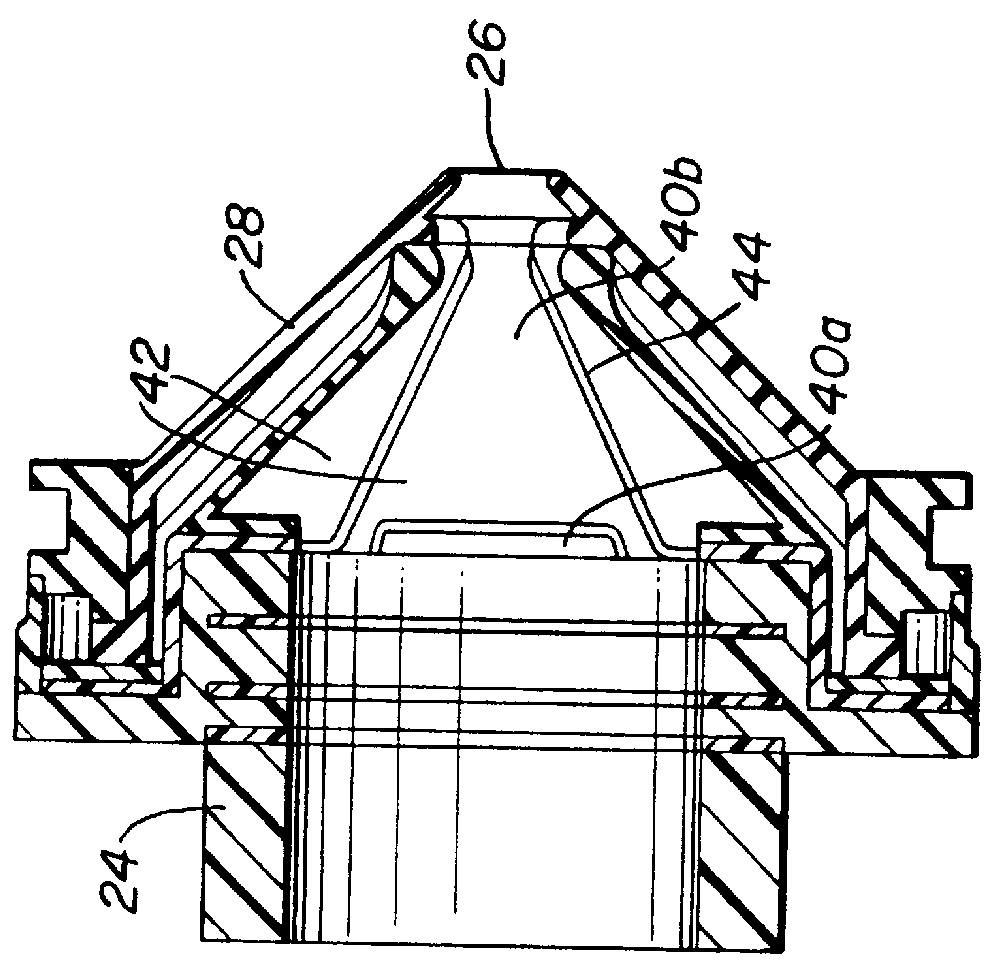
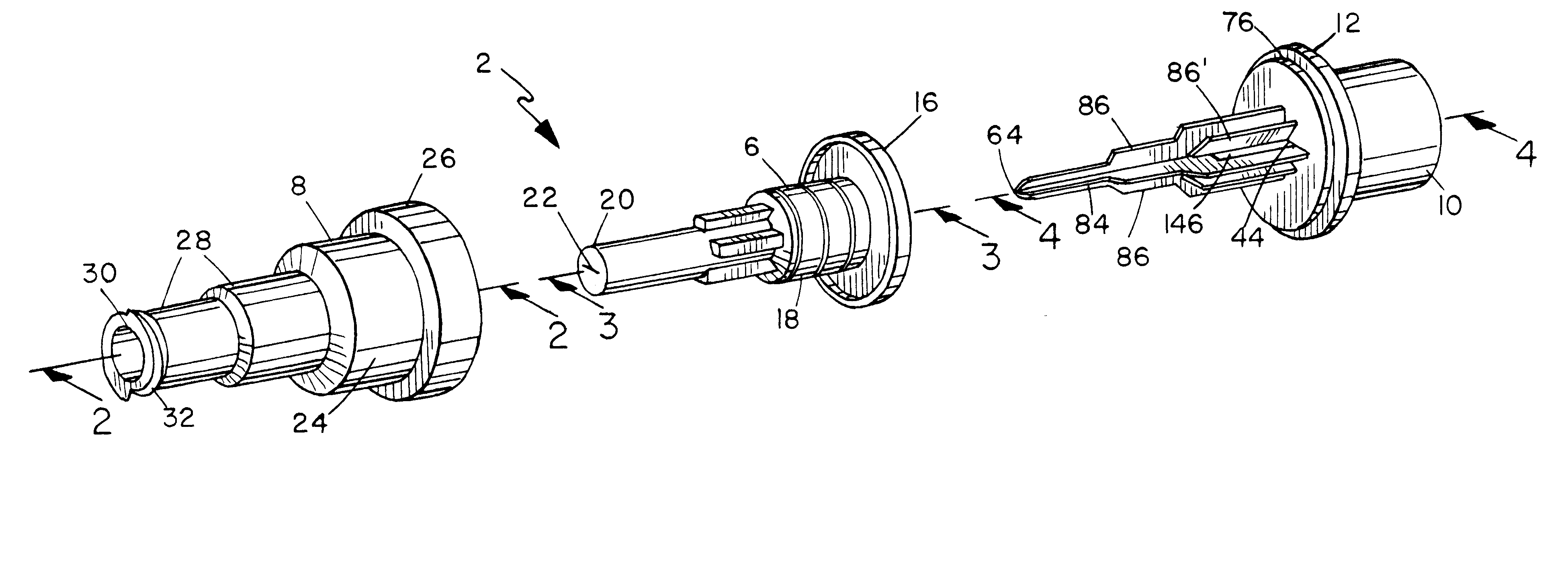
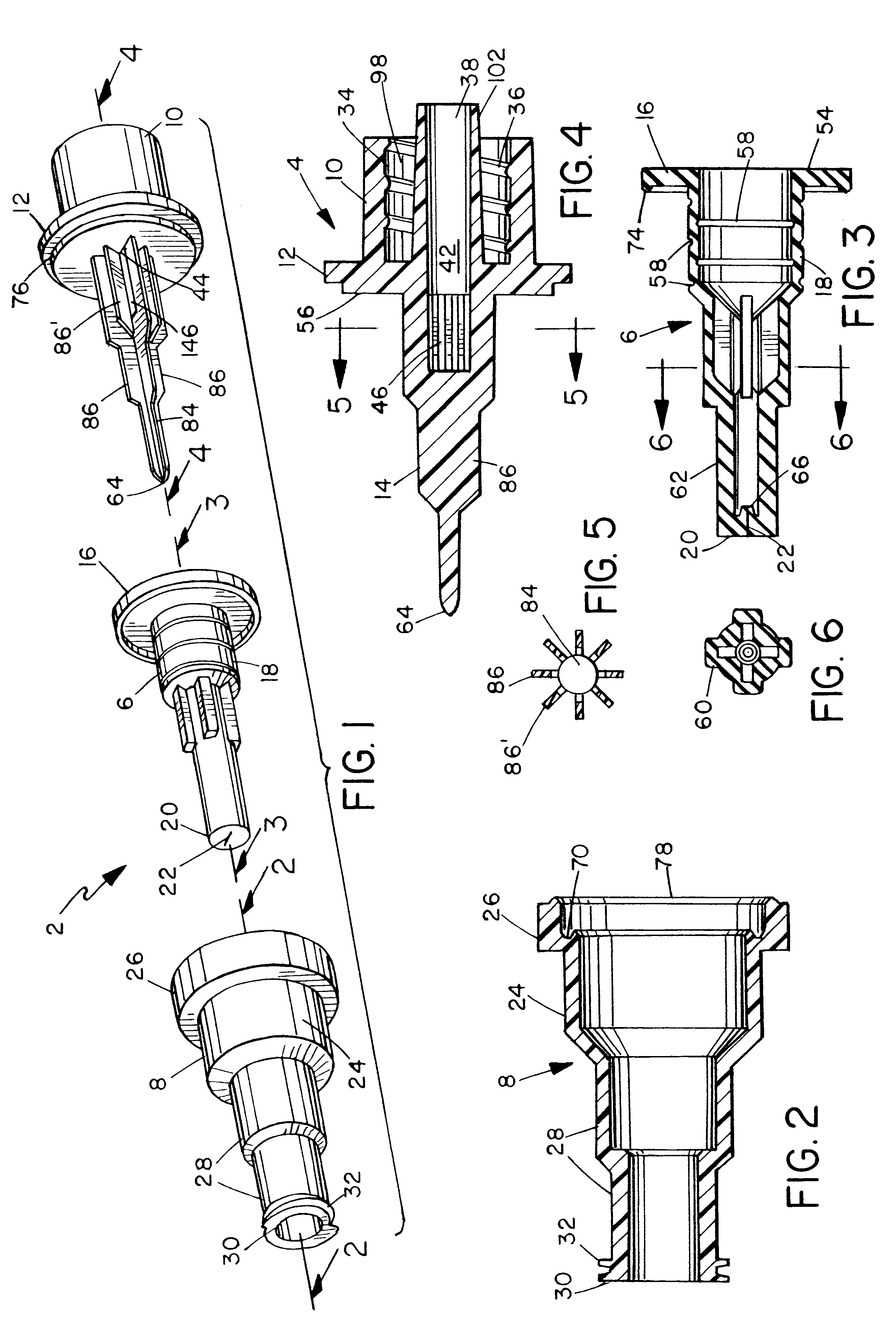
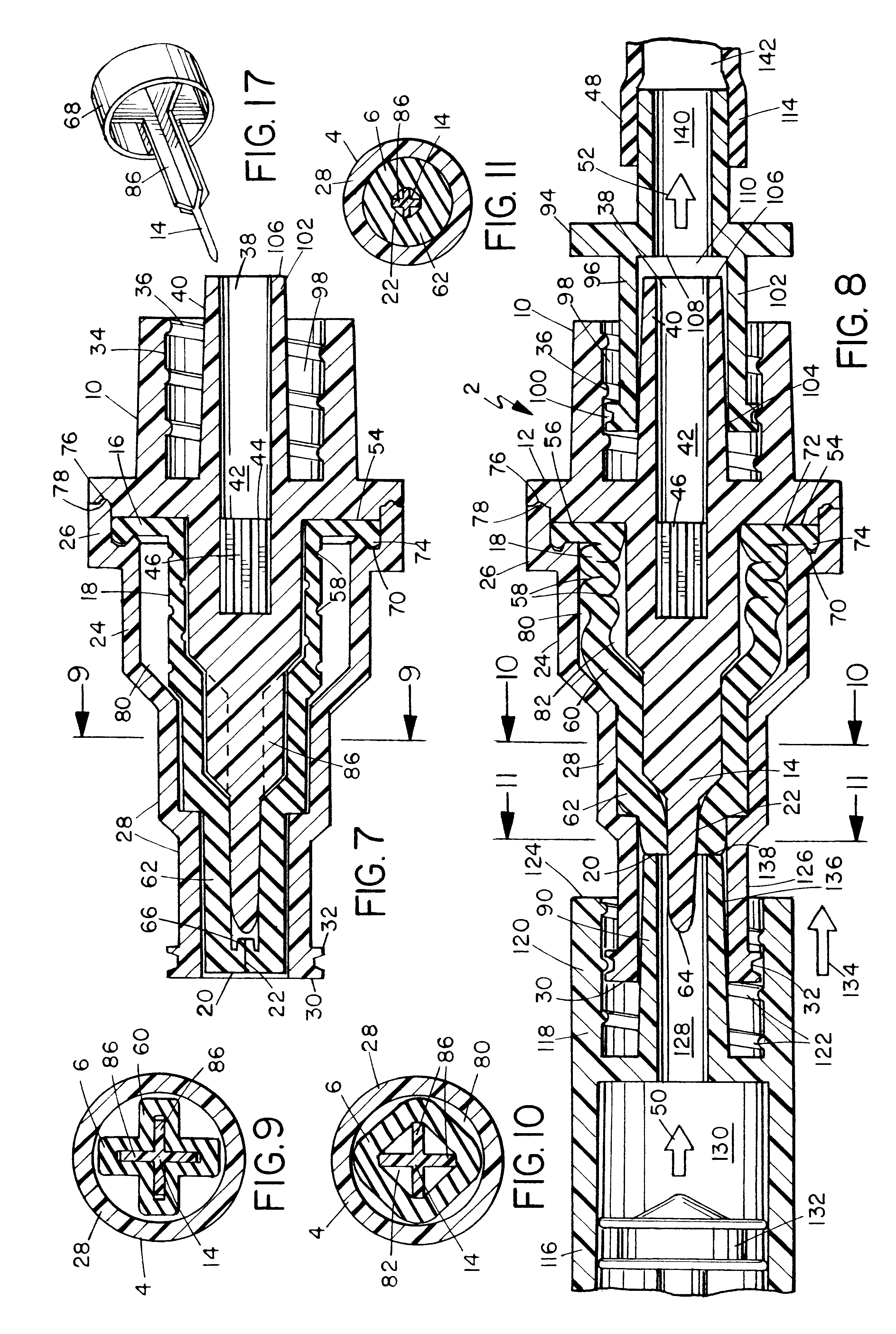
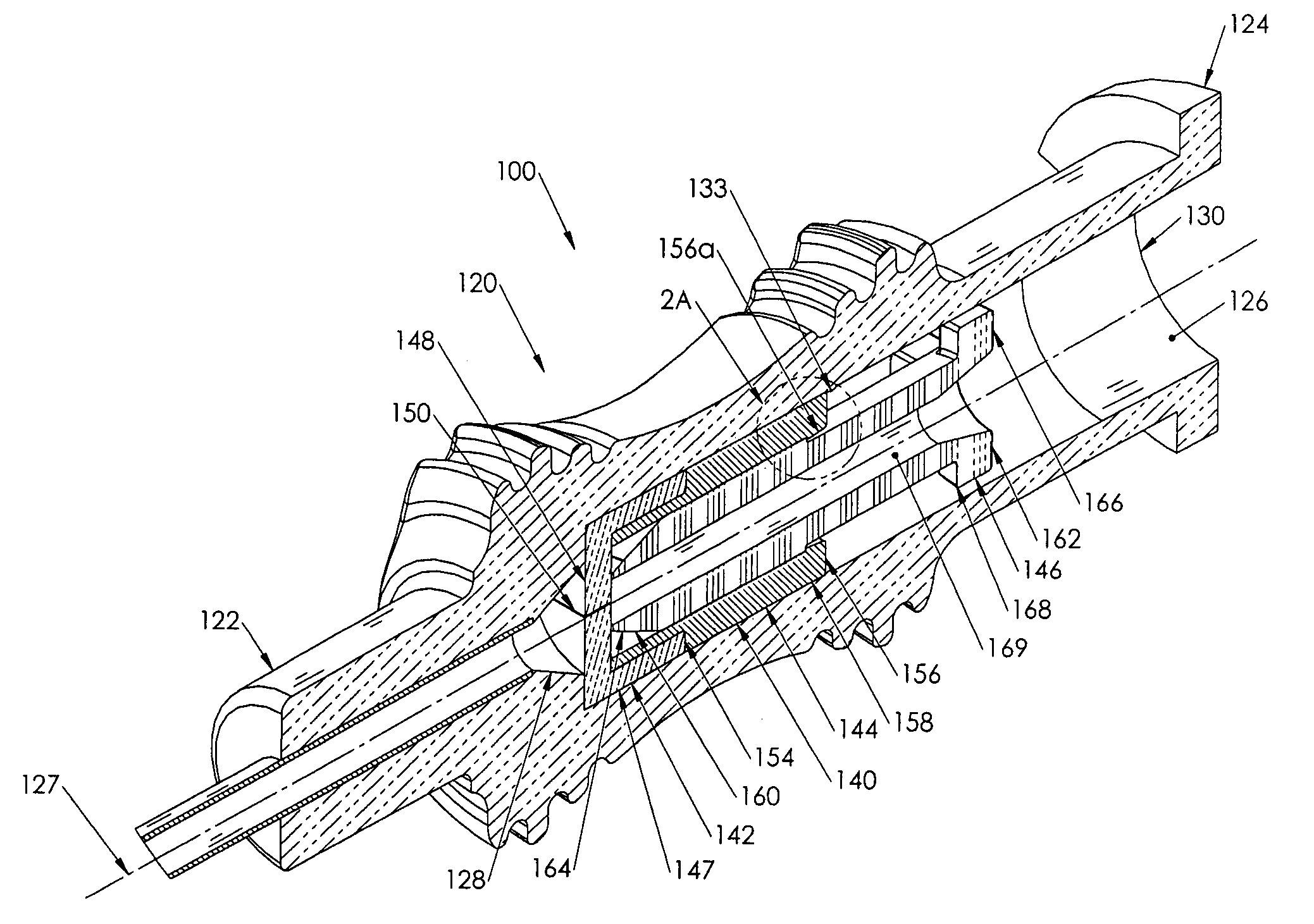
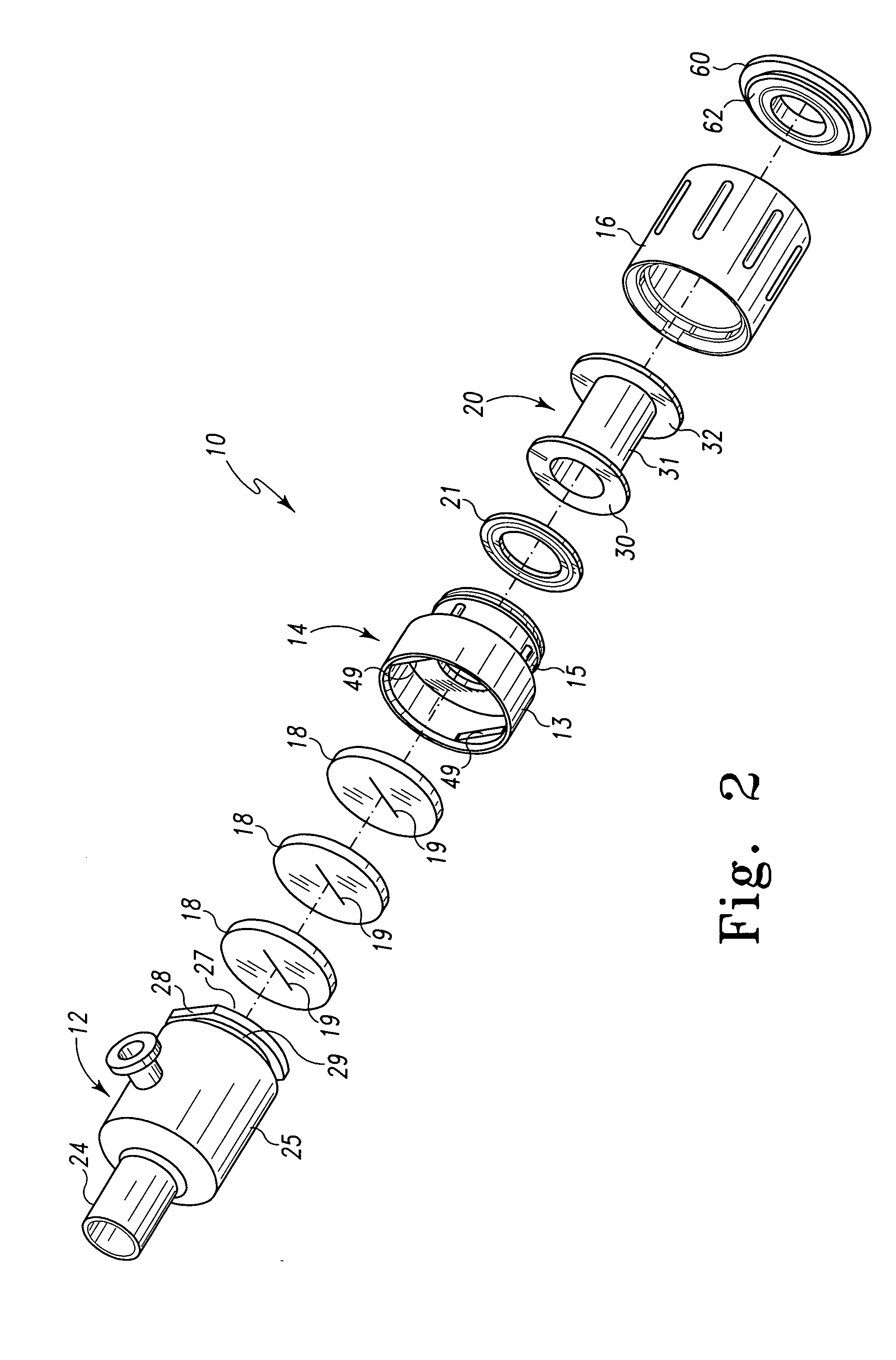
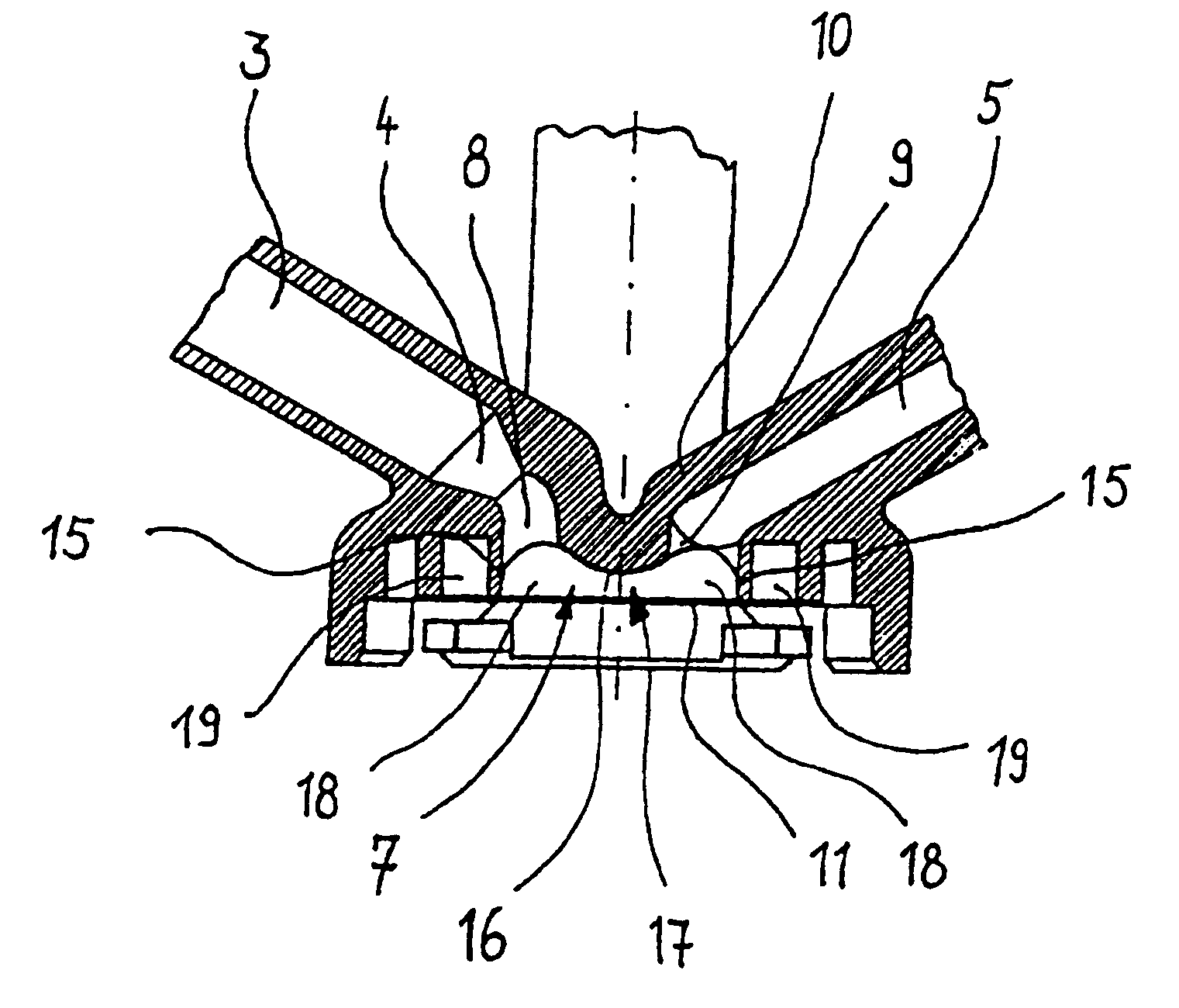
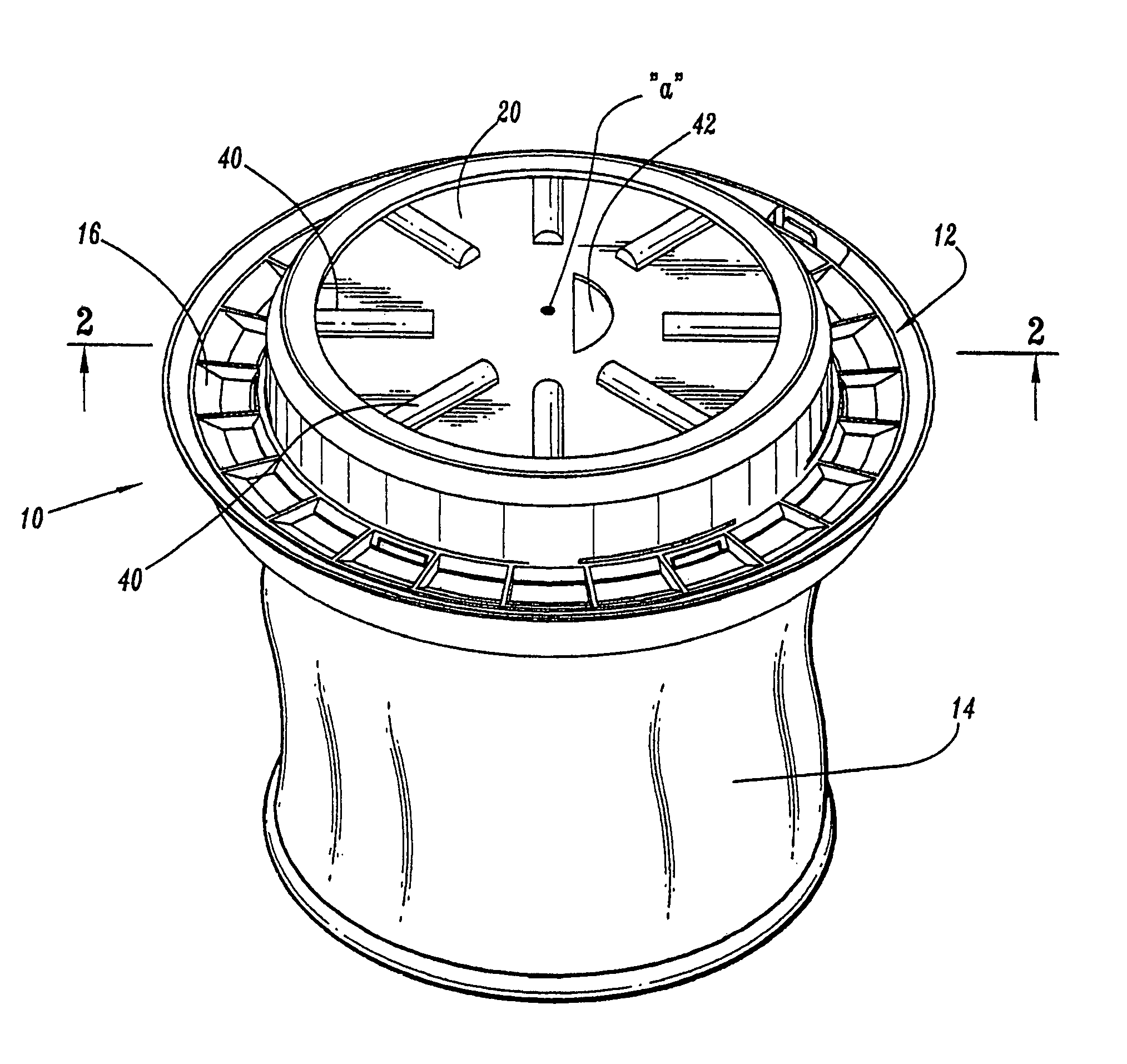
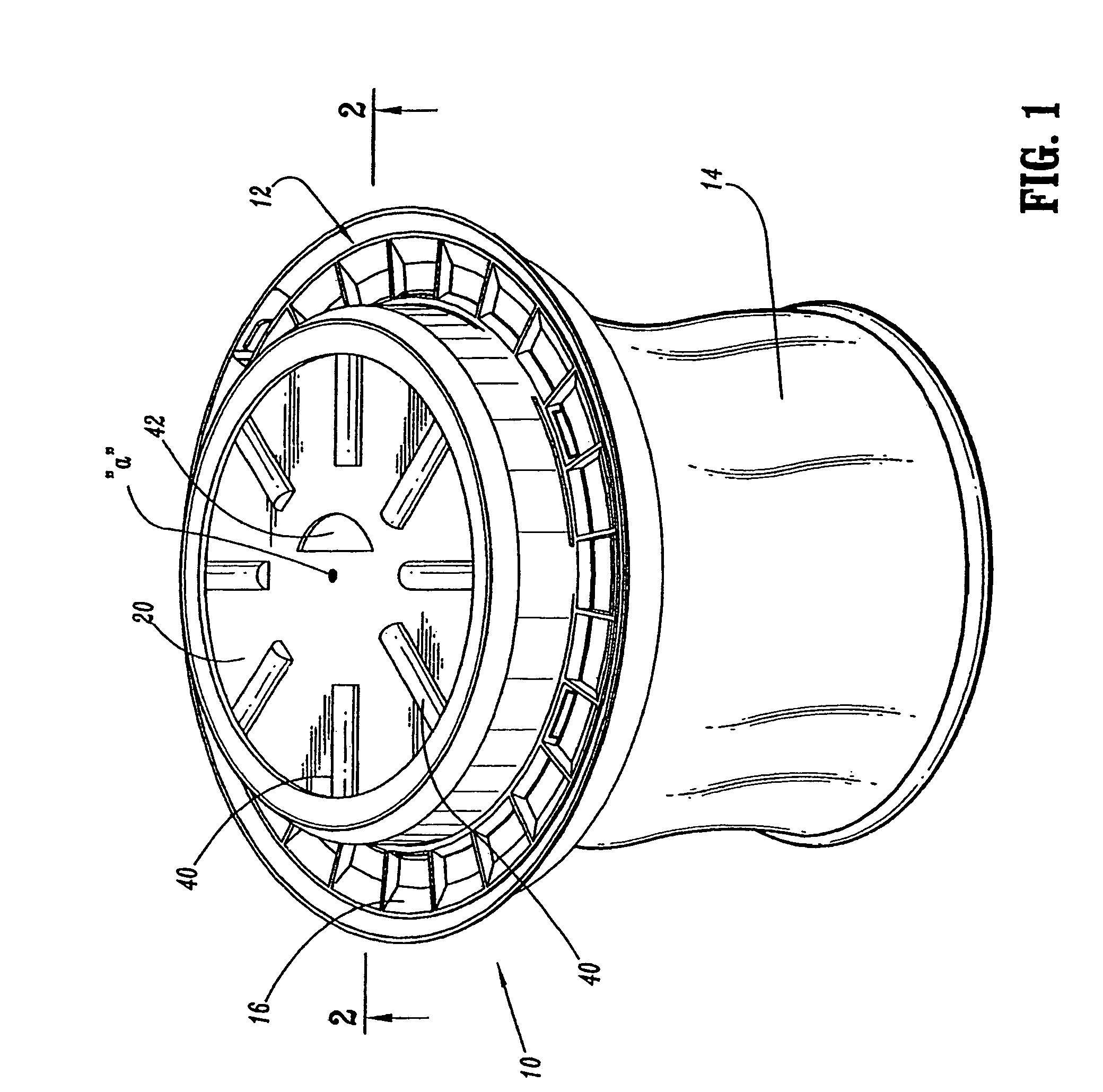
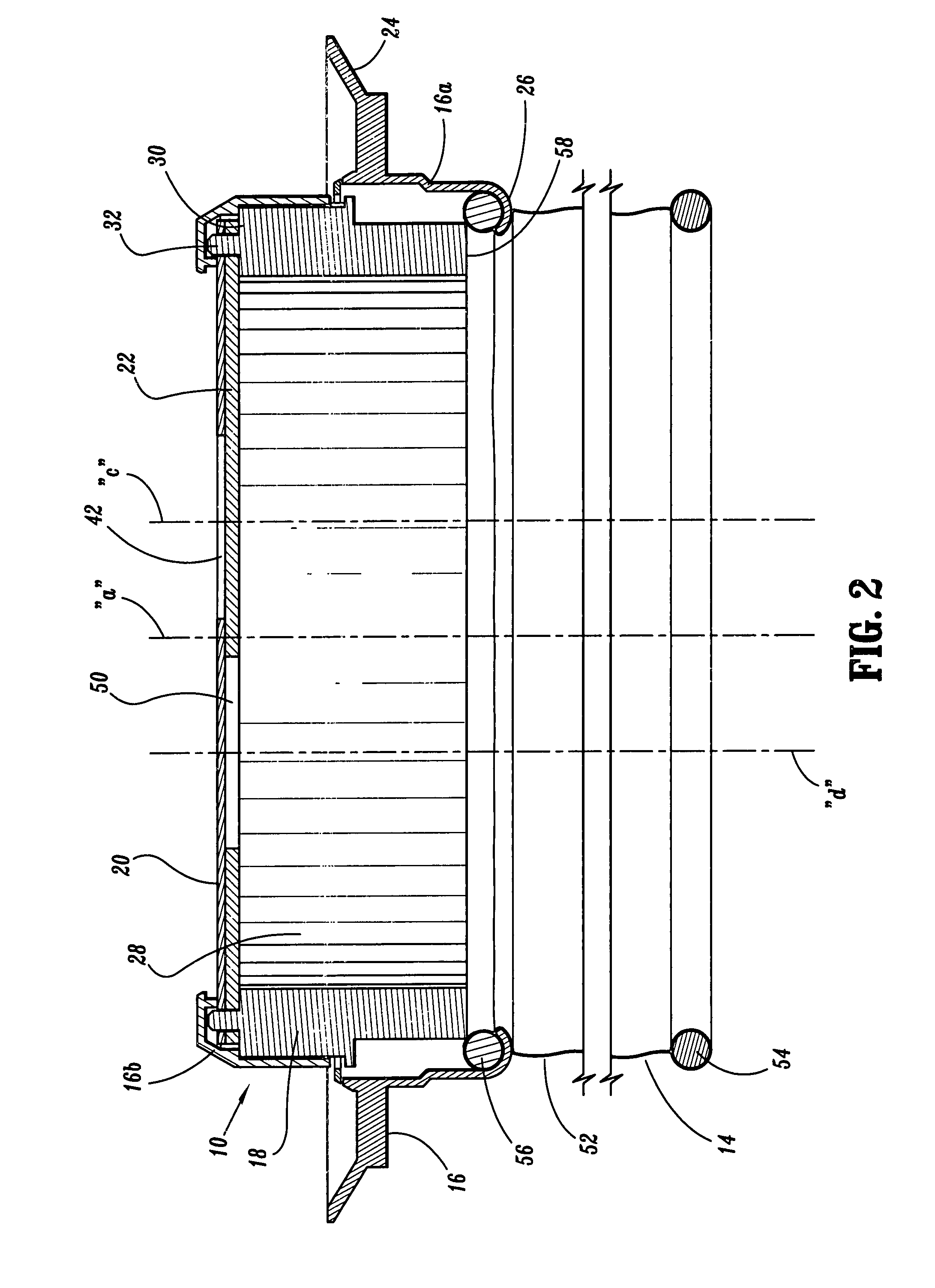
Surgical hand access apparatus
A surgical access apparatus adaptable to permit the sealed insertion of either the surgeon's hand and / or surgical instruments during laparoscopic and endoscopic surgical procedures includes an access member having a passageway therethrough and first and second seal members mounted across the passageway. The first and second seal members each has an aperture in non-overlapping relation to each other in the absence of the object positioned therein. The outer seal portion of the first seal member may include a plurality of expansible ribs and corresponding recesses arranged along respective lines of intersection with the axis of the first seal member.
Owner:COVIDIEN LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com