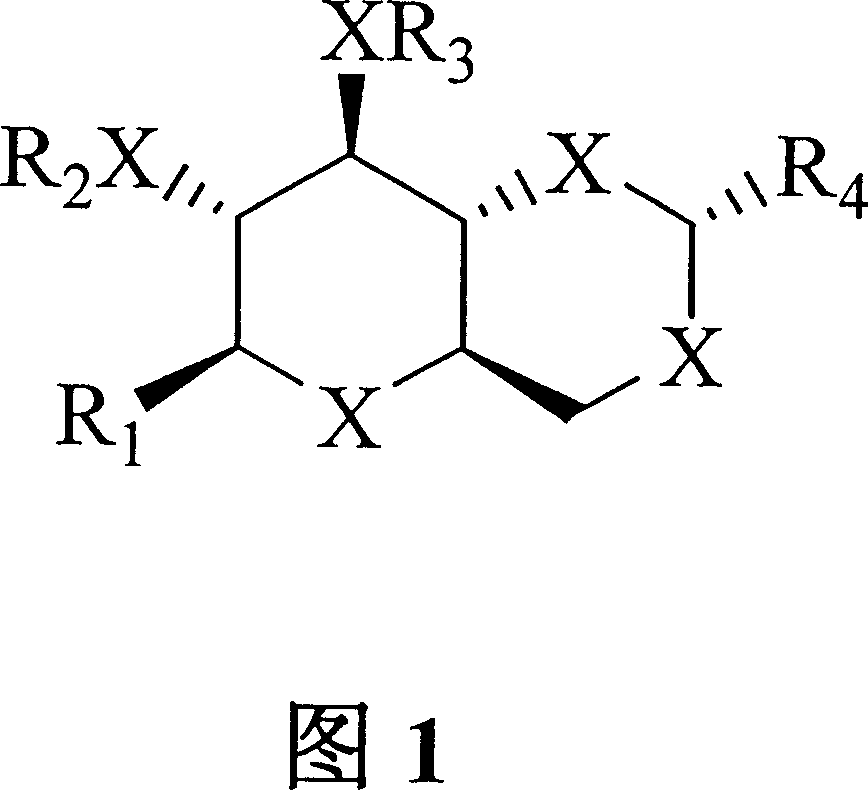
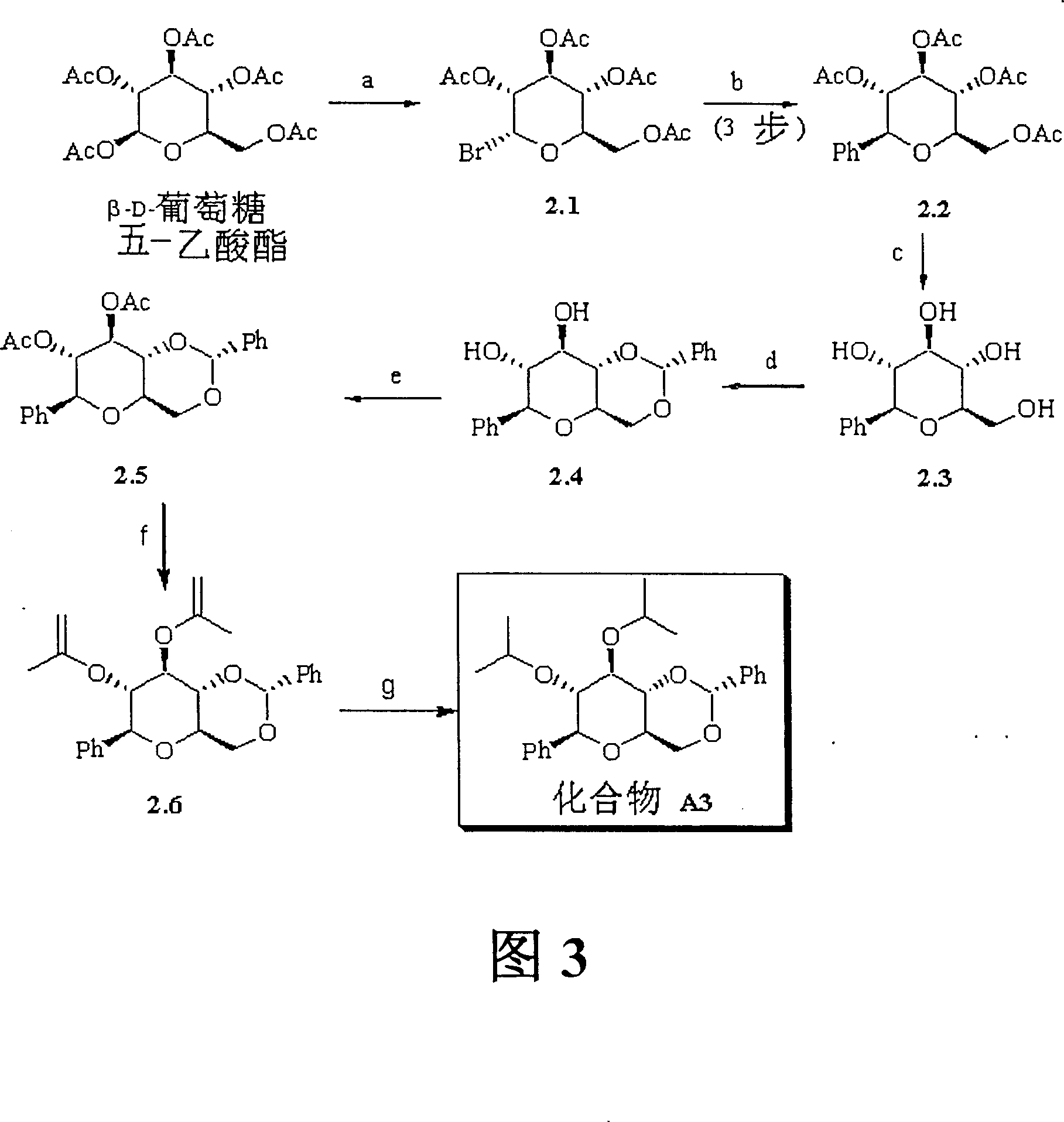
Bicyclic carbohydrate compounds useful in the treatment of infections caused by flaviviridae sp., such as hepatitis c and bovine viral diarrhea viruses
A technology of bovine viral diarrhea and compounds, applied in the direction of carbohydrate active ingredients, carbocyclyl sugars, sugar derivatives, etc., can solve the problems of lack of immunity and high proportion of re-infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0020] Methods and Materials
[0021] Synthesis of Formula A Compound
[0022] This compound is synthesized as follows:
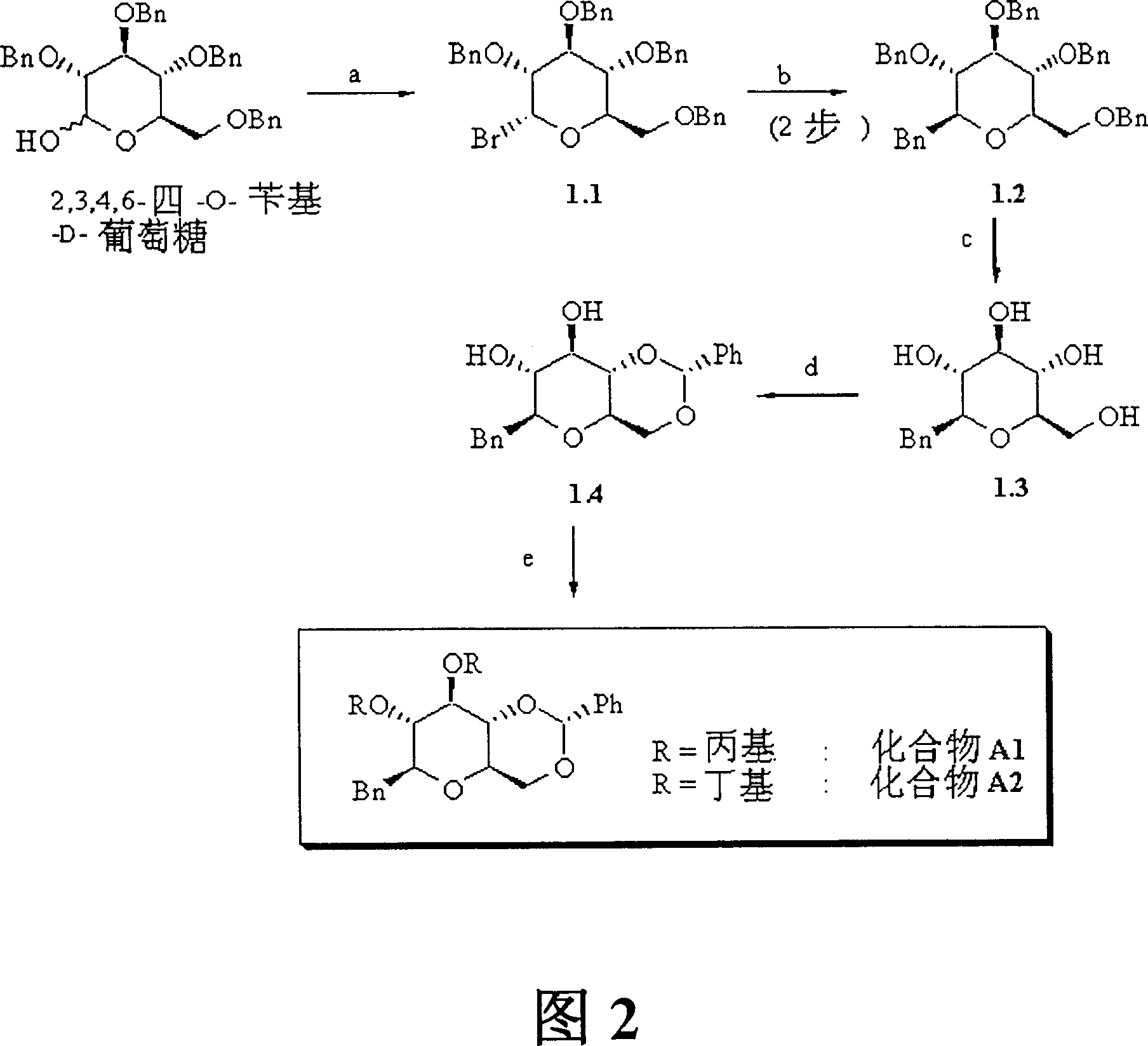
[0023] 1. Synthesis of compound A1 and compound A2
[0024] The synthesis of Compound A1 and Compound A2 is shown in Figure 2.
[0025] Synthesis of Compound 1.1
[0026] To 2,3,4,6-tetra-O-benzyl-D-glucose (10.0 g, 18.5 mmol) in CH at RT (room temperature) 2 Cl 2 (125ml) and DMF (6.25ml) were added dropwise to a solution of oxalyl bromide (2.5ml in CH 2 Cl 2 10M solution in , 1.35eq). Simultaneously with strong gas generation. The reaction mixture was stirred at room temperature under Ar atmosphere for 60 minutes. The reaction mixture was then poured into ice water (125ml). After phase separation, the organic phase was washed with ice water (2 x 125 ml). with MgSO 4 After drying, filtration and evaporation in vacuo, compound 1.1 was obtained as a yellow oil (Figure 1), which was used in the following reaction step without further purification. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap