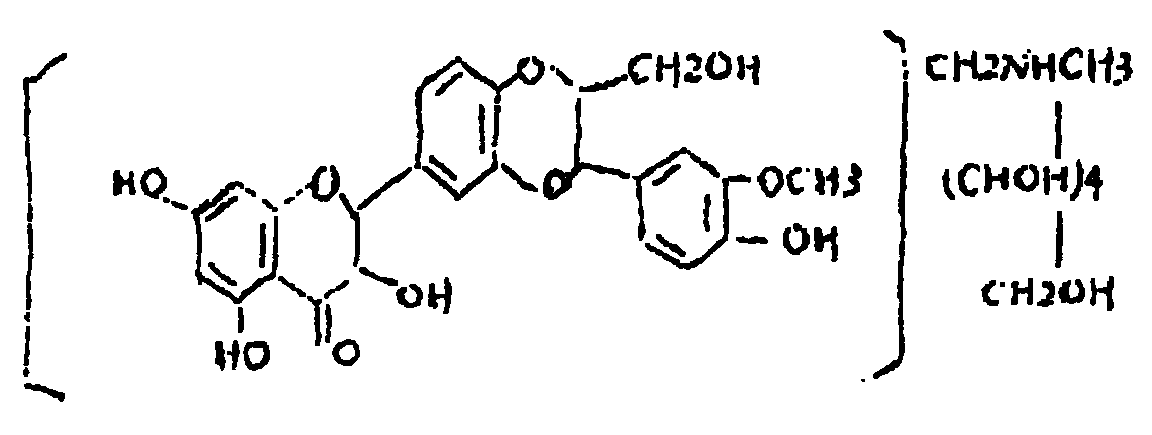
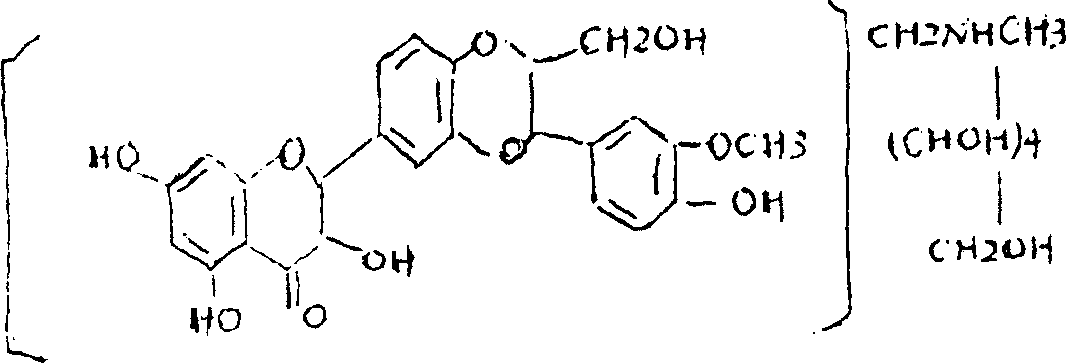
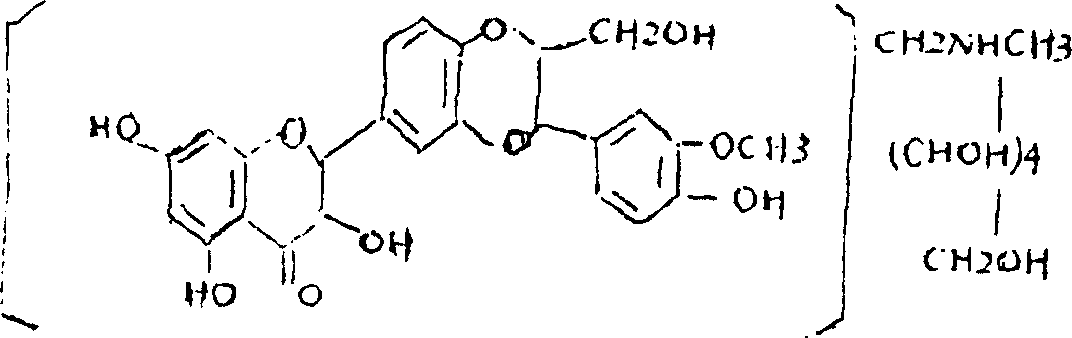
Silicibinin-N-methylglucamine disperser for treating hepatitis, and its prepn. method
A technology of silibinin and meglumine, which is applied in the field of medicine, can solve the problems of serious side effects, large dosage required, poor dissolution in water, etc., and achieves the effects of high bioavailability, small adverse reactions, and convenient administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] First, 100 grams of main drug silybin meglumine salt (<100 μm) was processed with all auxiliary materials except 6 grams of sodium starch glycolate such as 54.4 grams of lactose, 20 grams of mannitol, 1 gram of sodium carboxymethylcellulose, 3 G hydroxypropyl methylcellulose, 5 g microcrystalline cellulose, 10 g polyvinylpyrrolidone, 0.05 g sodium cetyl sulfosuccinate, 0.05 g sodium lauryl sulfate and 0.5 g magnesium stearate are mixed and pulverized , passed through a sieve below 30 meshes, then mixed evenly with sodium carboxymethyl starch, and pressed into tablets. The total weight of the ten auxiliary materials is 100 grams, and 1000 tablets are made, each auxiliary material is 100 mg, and the main drug is 100 mg, that is, the weight of the dispersible tablet is 200 mg.
Embodiment 2
[0032] 200 grams of main drug replace the main drug of embodiment 1, and the dosage and preparation method of all the other ten kinds of auxiliary materials are all the same as that of embodiment 1, then the weight of dispersible tablet is 300 mg, and each main drug is 200 mg.
Embodiment 3
[0034] Main drug 500 grams replace the main drug of embodiment 1, and all the other are the same as embodiment 1 or 2, then dispersible tablet weighs 600 mg, and each contains 500 mg of main drug. The embodiment of the present invention contains 100 mg, 200 mg, and 500 mg / tablet of the main drug in three specifications, and other embodiments containing 50-1000 mg / tablet of the main drug, the method is the same as above.
[0035] The product of the invention mainly treats acute hepatitis, chronic hepatitis, primary liver cirrhosis, fatty liver, and liver damage caused by toxicity (such as taking drugs that damage liver cells or drinking a lot of alcohol frequently) and protecting the liver.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com