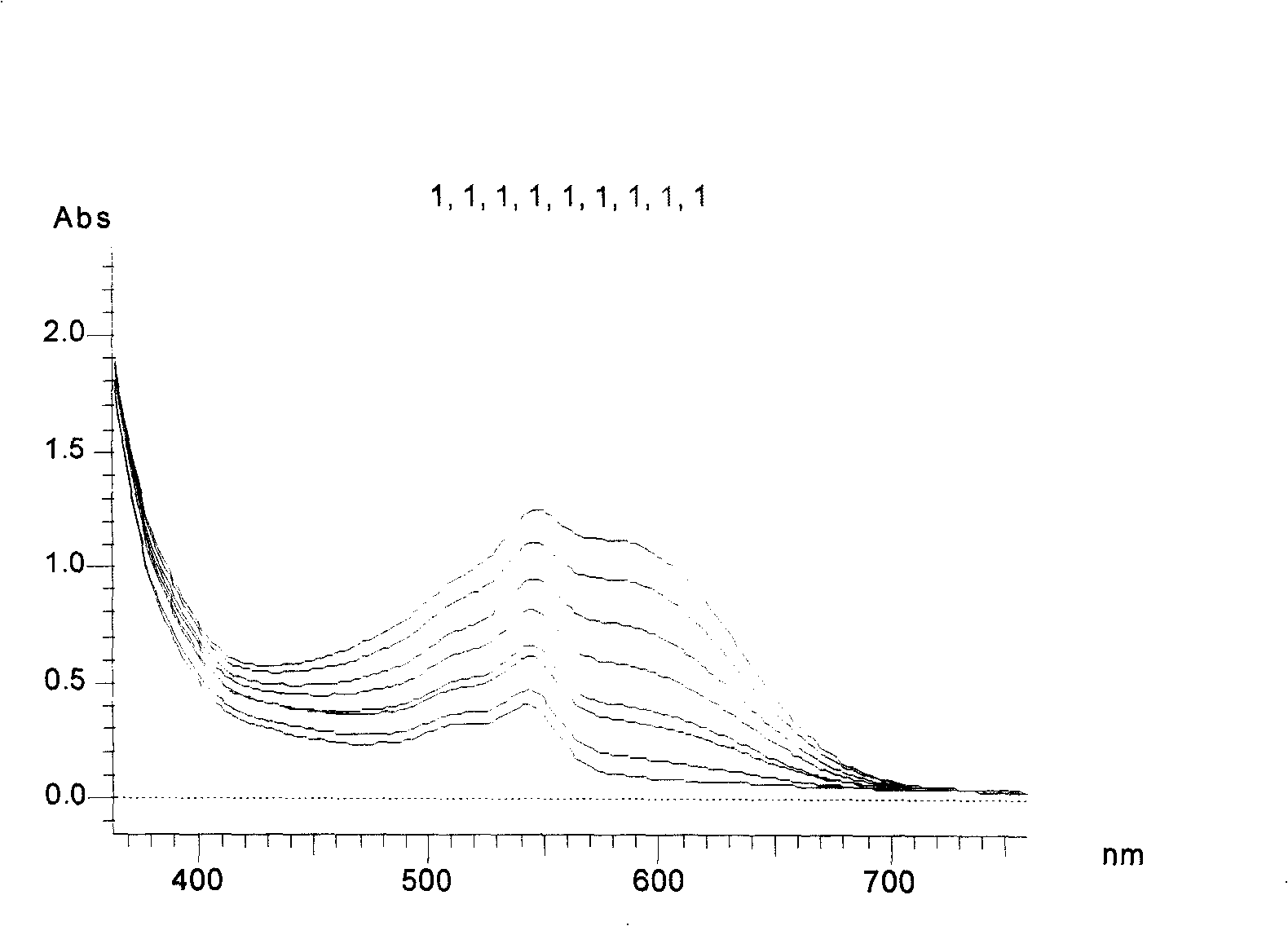
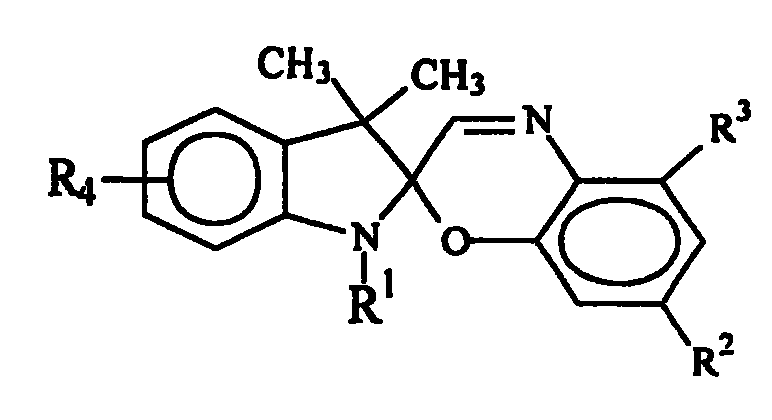
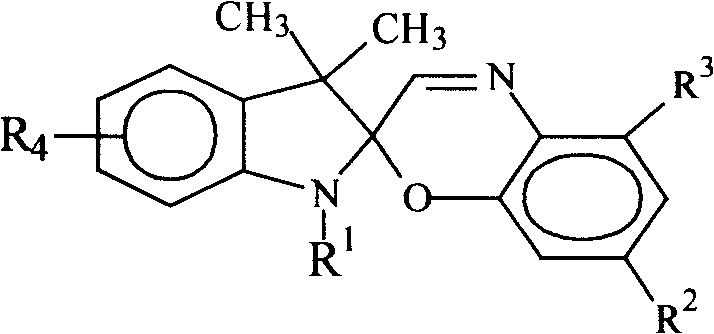
Indolinospirobenzoxazine compound, and synthesis method and use thereof
An indoline spiro and synthetic method technology, which is applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., to achieve the effects of increasing selectivity and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of 1,3.3-trimethyl-7'-n-butyl, 5'-pyrrolidinyl-indoline[2,3']spirobenzo[1,4]oxazine:
[0042] Under nitrogen protection, according to literature [E.FICHER; SCHMITT, BER, 21,1072 (1888) method preparation 1,2,3,3-tetramethyl-indolenine iodonium salt is dissolved in absolute ethanol solution, Add 5wt% catalyst hexahydropyridine, under reflux conditions, drop 2-nitroso-3-n-butoxy- The dehydrated ethanol solution of 5-tetrahydropyrrolyl-phenol is distilled while being added dropwise, and the rate of addition is equal to the rate of solvent distillation. After the dropwise addition is completed. Continue to reflux for 1-2 hours. The solution was distilled, concentrated, and passed through a silica gel column to obtain the product indole spirobenzoxazine compound. 1 HNMR (CDCl 3 ), δ (ppm): 7.45-6.5 (m, 4H,), 5.66 (s, 1H,), 5.57 (s, 1H, Ar-H), 4.1 (t, 2H, OCH 2 ,), 3.32(t, 4H, CH 2 -N-CH 2 ), 2.8(s, 3H, N-CH 3 )2.0(t,4H,N-CH 2 -), 1.8 (p, 2H, -CH 2 -), 1.5...
Embodiment 2
[0044] Synthesis of 1,3,3-trimethyl-5',7'-dimorpholinoindoline-[2,3'], spirobenzo[1,4]oxazine:
[0045] Under nitrogen protection, according to literature E.FICHER; SCHMITT, BER, 21,1072 (1888) method prepared 1,2,3,3-tetramethyl-indolenine iodide salt was dissolved in dehydrated ethanol solution, added 5wt% catalyst hexahydropyridine, under reflux conditions, add dropwise 2-nitroso-3,5-dimorpholino with 1,2,3,3-tetramethyl-indolenine iodide -The dehydrated ethanol solution of phenol, distill while adding dropwise, and make the rate of addition equal to the rate of solvent distillation. After the dropwise addition is completed. Continue to reflux for 1-2 hours. After concentration, pass through a silica gel column to obtain the indole spirobenzoxazine compound, which is recrystallized in ethanol. 1 H NMR, (CDCl 3 ), δ (ppm): 7.4-6.4 (m,, 4H), 5.85 (s,, 1H), 5.95 (s, 1H), 3.85 (t, O-CH 2 , 8H), 3.6(t-N-CH 2 , 8H), 3.2(s, 3H, N-CH 3 )0.95(s, 6H, CH 3 ) has good photochro...
Embodiment 3
[0048] Synthesis of 1-propenyl-3,3-dimethyl-5',7'-dimorpholinoindoline[2,3']-spirobenzo[1,4]oxazine:
[0049] Under nitrogen protection, according to the document E.FICHER; SCHMITT, BER, 21,1072 (1888) method prepared 1-propenyl-2,3,3-trimethyl-indolenine iodonium salt was dissolved in absolute ethanol In the solution, add 5% catalyst hexahydropyridine, and under reflux conditions, add dropwise 2-nitroso- 3,5-Dimorpholino-phenol absolute ethanol, distill while adding dropwise, and make the dropping rate equal to the distillation rate. After the dropwise addition is completed. Continue to reflux for 1-2 hours. After concentration, pass through a silica gel column to obtain the target compound. MP: 59-60°C 1 HNMR (CDCl 3 ), δ (ppm): 1.33 (s, 6H, CH 3 ), 3.15(t, 8H, N-CH 2 ,), 3.25(t, 2H, N-CH 2 -), 3.82(t, O-CH 2 , 8H,), 5.16 (d, 2H, -CH=CH 2 ,), 5.76 (d, 1H, -CH=CH 2 ), 6.0 (2s, 2H, Ar-H), 6.58-7.46 (m,, 5H,). Has good photochromic properties and stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com