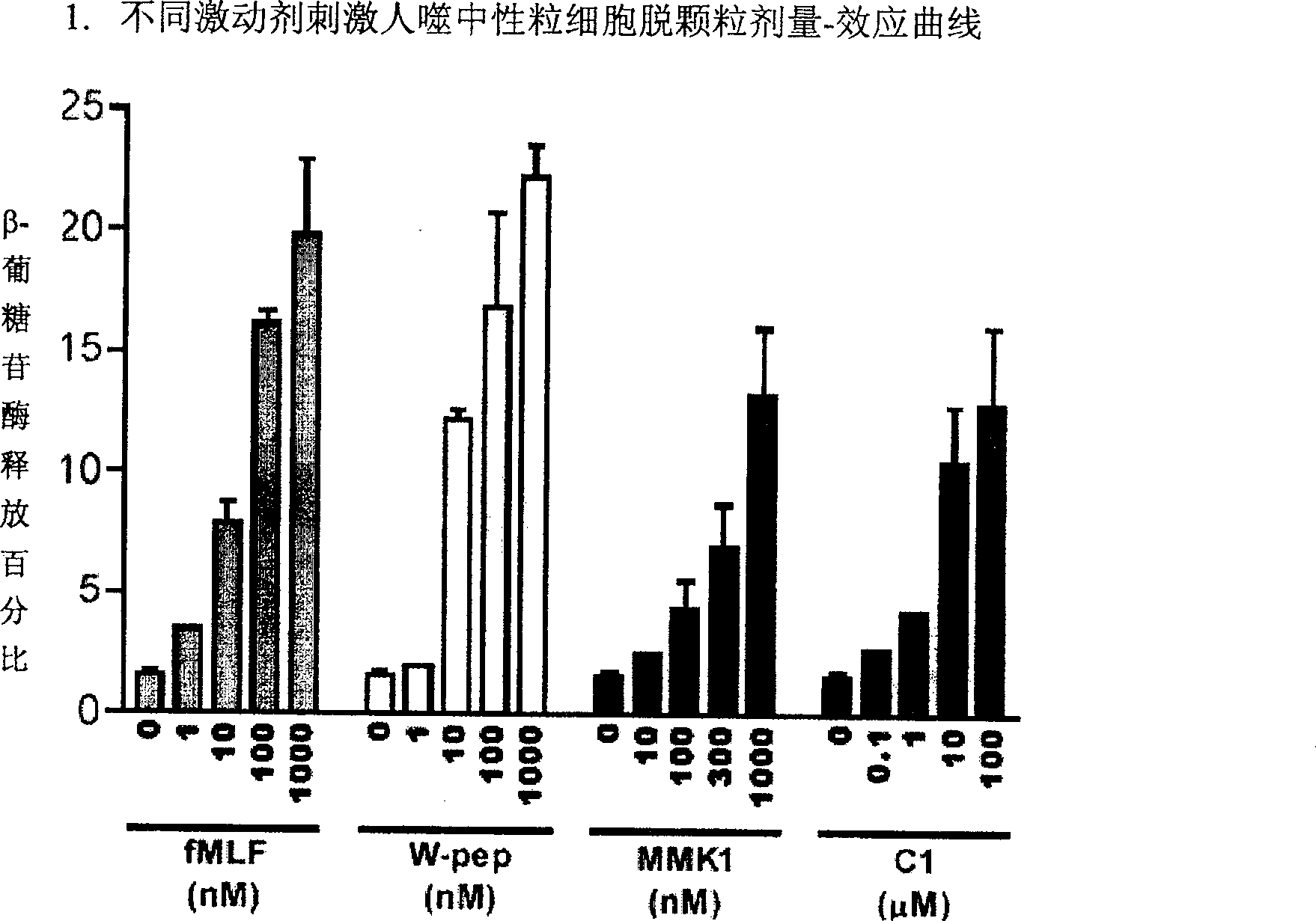
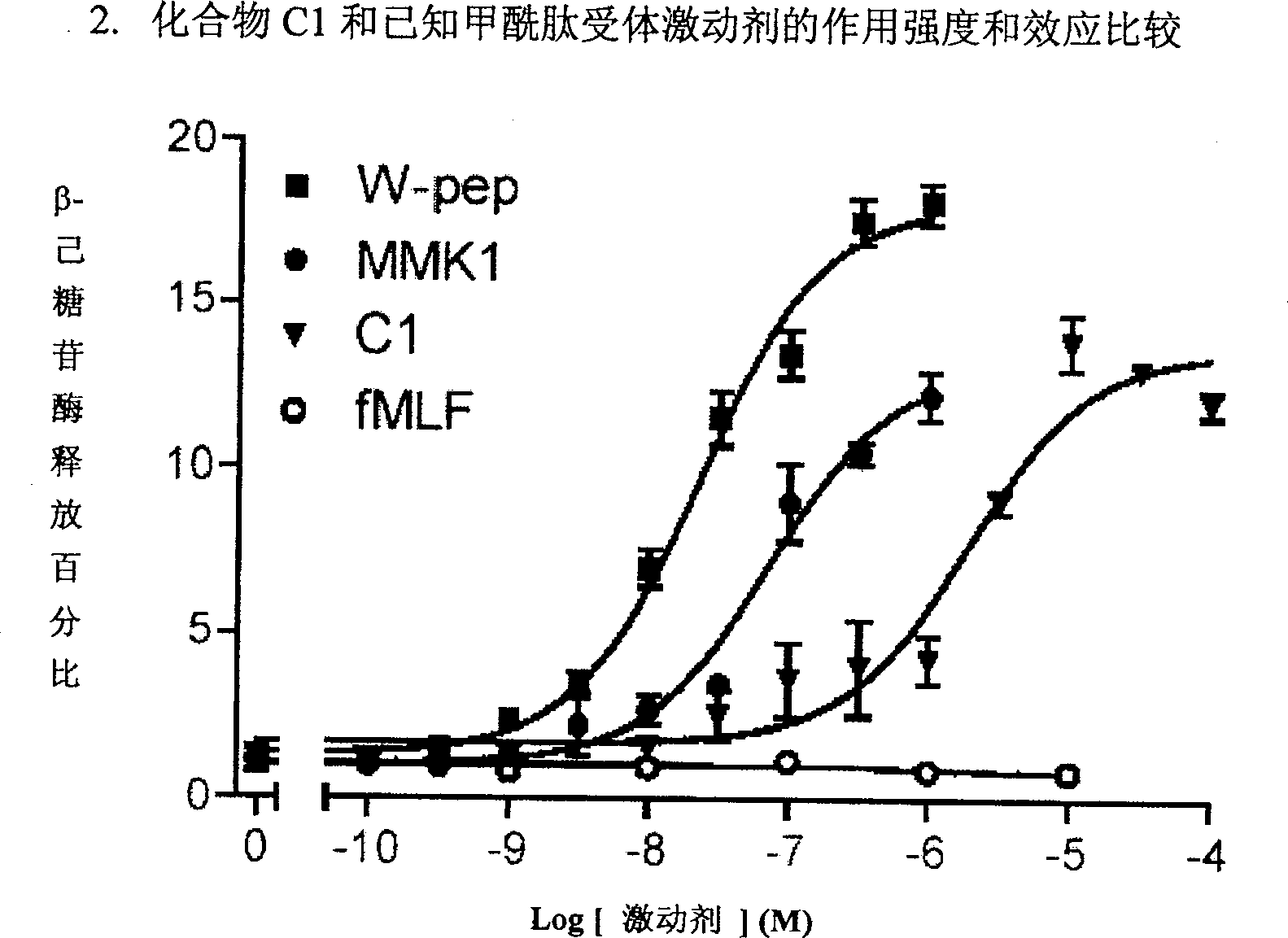
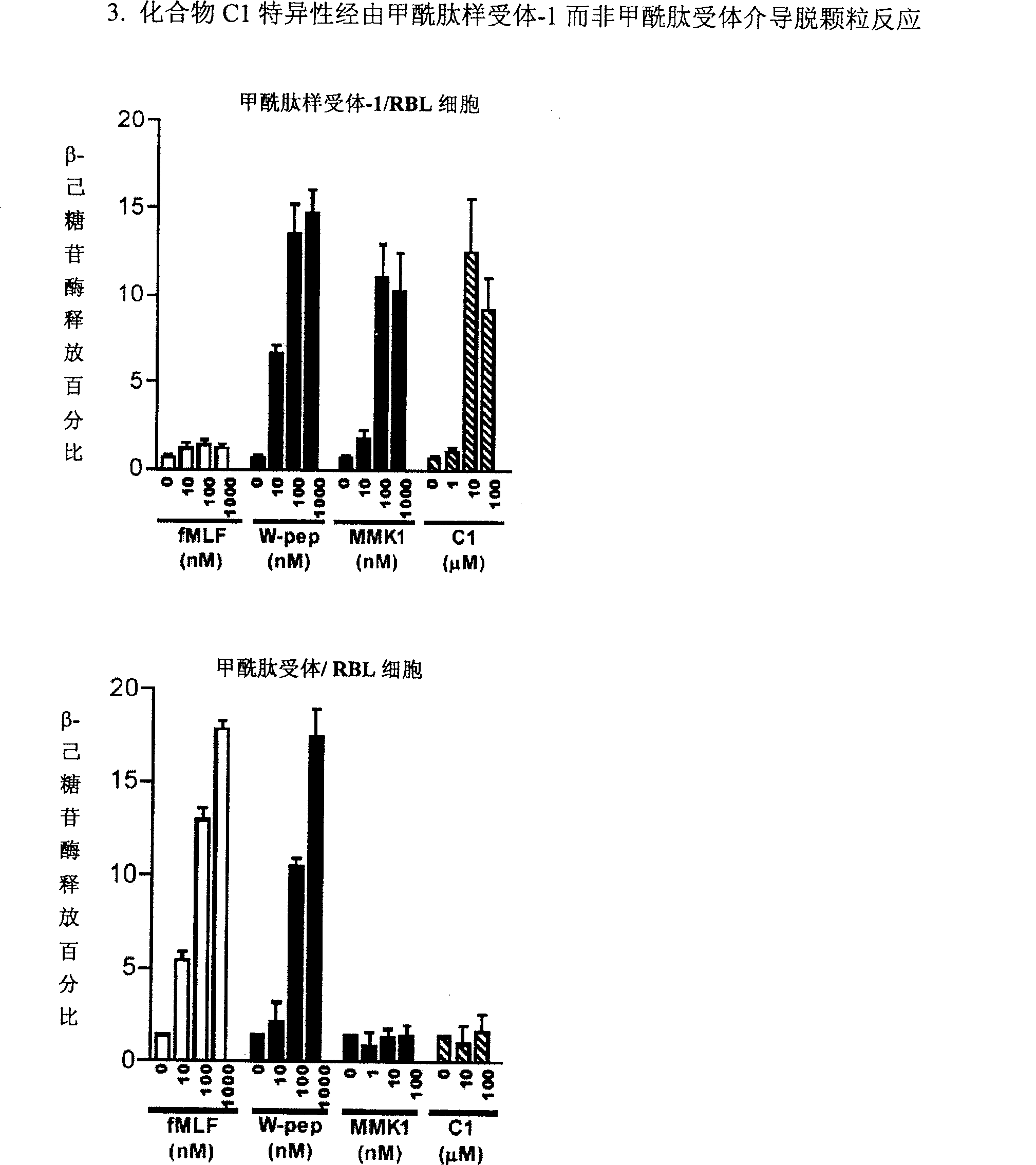
Formyl peptide-like acceptor-1 regulator and its Preparation and use
A technology of benzamide and methyl group is applied in the field of formyl peptide-like receptor-1 modulators, which can solve the problems such as the discovery of organic small molecule ligands for specific FPRL1 receptors that have not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Dissolve 0.30g of N-(4-butoxy-benzoyl)-2-amino-benzohydrazide (1) in 10ml of N,N-dimethylacetamide containing 5% glacial acetic acid, add 0.25 g 4-methoxybenzaldehyde, heated at 80°C for 24 hours. After the reaction was completed, the reaction solvent was removed, and the product 2-(4-methoxy-phenyl)-2,3-dihydro-3-(4-butoxy-benzamide)- 1H-Quinazolin-4-one (C1).
[0033] 1 HNMR (300MHz, DMSO-d 6 )δ 0.92(t, 3H), 1.41(m, 2H), 1.67(m, 2H), 3.73(s, 3H), 3.99(t, 2H), 6.11(s, 1H), 6.75(m, 2H) , 6.91 (m, 4H), 7.33-7.68 (m, 6H).
Embodiment 2
[0035]
[0036] 0.30g N-(4-butoxy-benzoyl)-2-amino-benzohydrazide (1) and 0.30g 2,4-dimethoxybenzaldehyde in 10ml of N containing 5% glacial acetic acid, In N-dimethylacetamide, heat the reaction at 50°C for 24 hours to obtain the product 2-(2,4-dimethoxy-phenyl)-2,3-dihydro-3-(4-butane Oxy-benzamide)-1H-quinazolin-4-one (C2).
[0037] 1 HNMR (300MHz, DMSO-d 6 )δ 0.92(t, 3H), 1.42(m, 2H), 1.68(m, 2H), 3.59(s, 3H), 3.72(s, 3H), 4.00(t, 2H), 6.40(s, 1H) , 6.52 (m, 2H), 6.73 (m, 2H), 6.94-7.02 (m, 3H), 7.28 (t, 1H), 7.48 (m, 1H), 7.65 (m, 2H).
Embodiment 3
[0039]
[0040] 0.30g N-(4-butoxy-benzoyl)-2-amino-benzohydrazide (1) and 0.27g 4-dimethylaminobenzaldehyde in 10ml containing 5% glacial acetic acid N, N-di In methylacetamide, heated at 60°C for 24 hours to obtain the product 2-(4-dimethylamino-phenyl)-2,3-dihydro-3-(4-butoxy-benzamide) -1H-quinazolin-4-one (C3).
[0041] 1 HNMR (300MHz, DMSO-d 6 )δ 0.92(t, 3H), 1.42(m, 2H), 1.68(m, 2H), 2.84(s, 6H), 4.00(t, 2H), 6.04(s, 1H), 6.68-6.79(m, 4H), 6.92 (m, 2H), 7.17-7.30 (m, 4H), 7.61 (m, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com