Bacteria count culture medium for limit test of microbe in medication of quinolone category, and application
A microbial limit, quinolone technology, applied in the field of microbial culture medium engineering, can solve the problems of polluting microorganisms, difficult to remove, invalid test results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
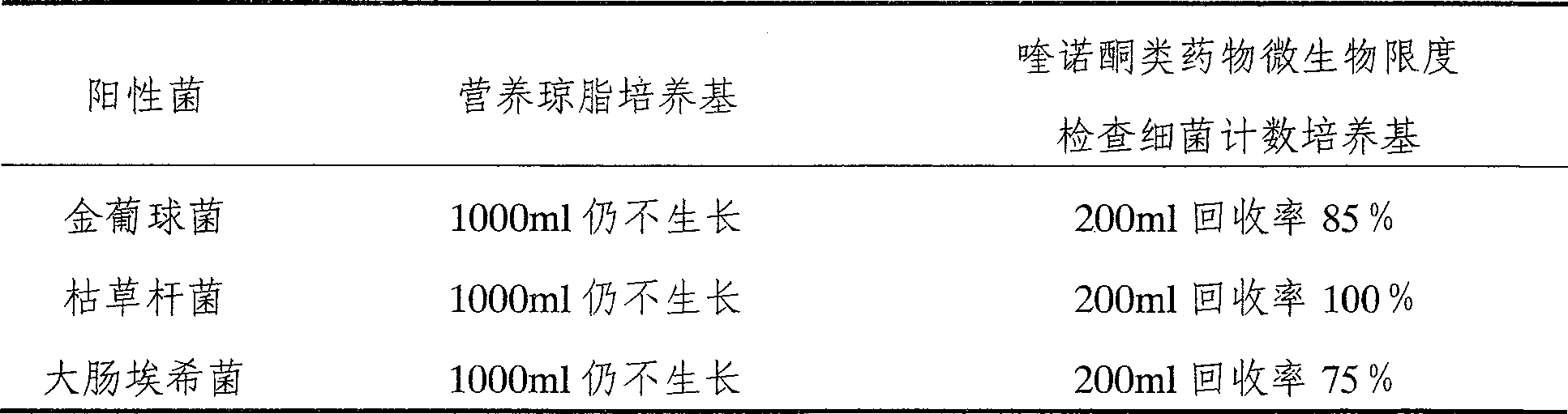
[0024] Quinolones microbial limit test bacteria count medium is: peptone 10.0g, beef extract powder 5.0g, sodium chloride 5.0g, agar 14.0g, manganese sulfate (MnSO 4 ·H 2 O) 4.2g, water 1000ml, adjust pH7.1.
[0025] Preparation process: Weigh (quantify) each component sequentially according to the above formula, add 900ml of water to dissolve, adjust the pH to 7.1 with 1mol / L NaOH solution, and then add water to 1000ml. The same below.
Embodiment 2
[0027] Preparation process is the same as embodiment 1, and the difference is that the quinolones drug microbial limit inspection bacteria count medium is: peptone 10.0g, beef extract powder 5.0g, sodium chloride 5.0g, agar 14.0g, manganese sulfate (MnSO 4 ·H 2 O) 0.845g, water 1000ml, adjust pH6.9.
Embodiment 3
[0029] Preparation process is the same as embodiment 1, and the difference is that the quinolones drug microbial limit inspection bacteria count medium is: peptone 10.0g, beef extract powder 5.0g, sodium chloride 5.0g, agar 14.0g, manganese sulfate (MnSO 4 ·H 2 O) 16.9g, water 1000ml, adjust pH7.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com