Compositions for delivering hypnotic agents across the oral mucosa and methods of use thereof
A technology for compositions and medicines, which can be used in pharmaceutical formulations, powder delivery, pill delivery, etc., and can solve problems such as the ability to trouble the final amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
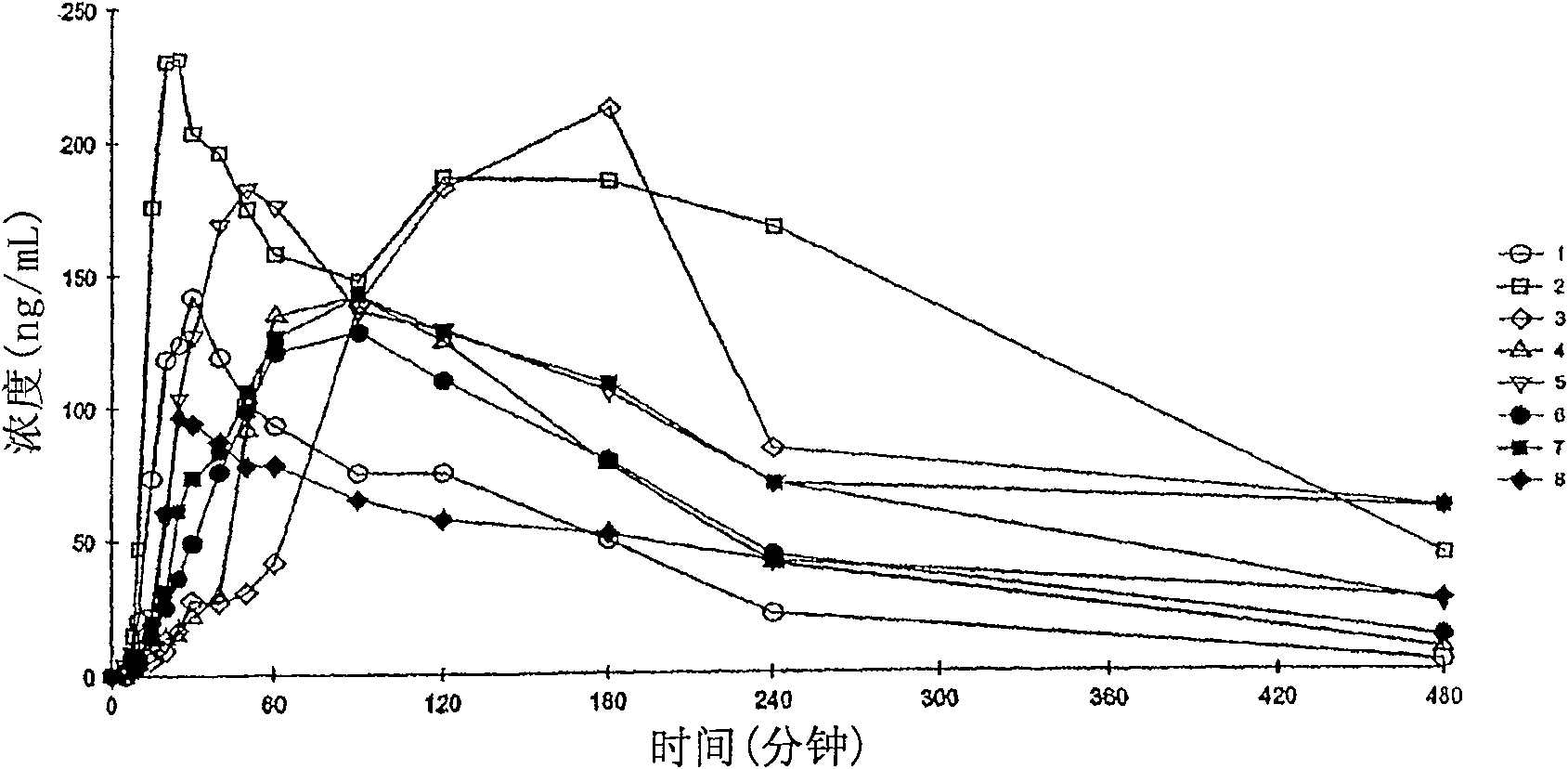
Examples
Embodiment 1
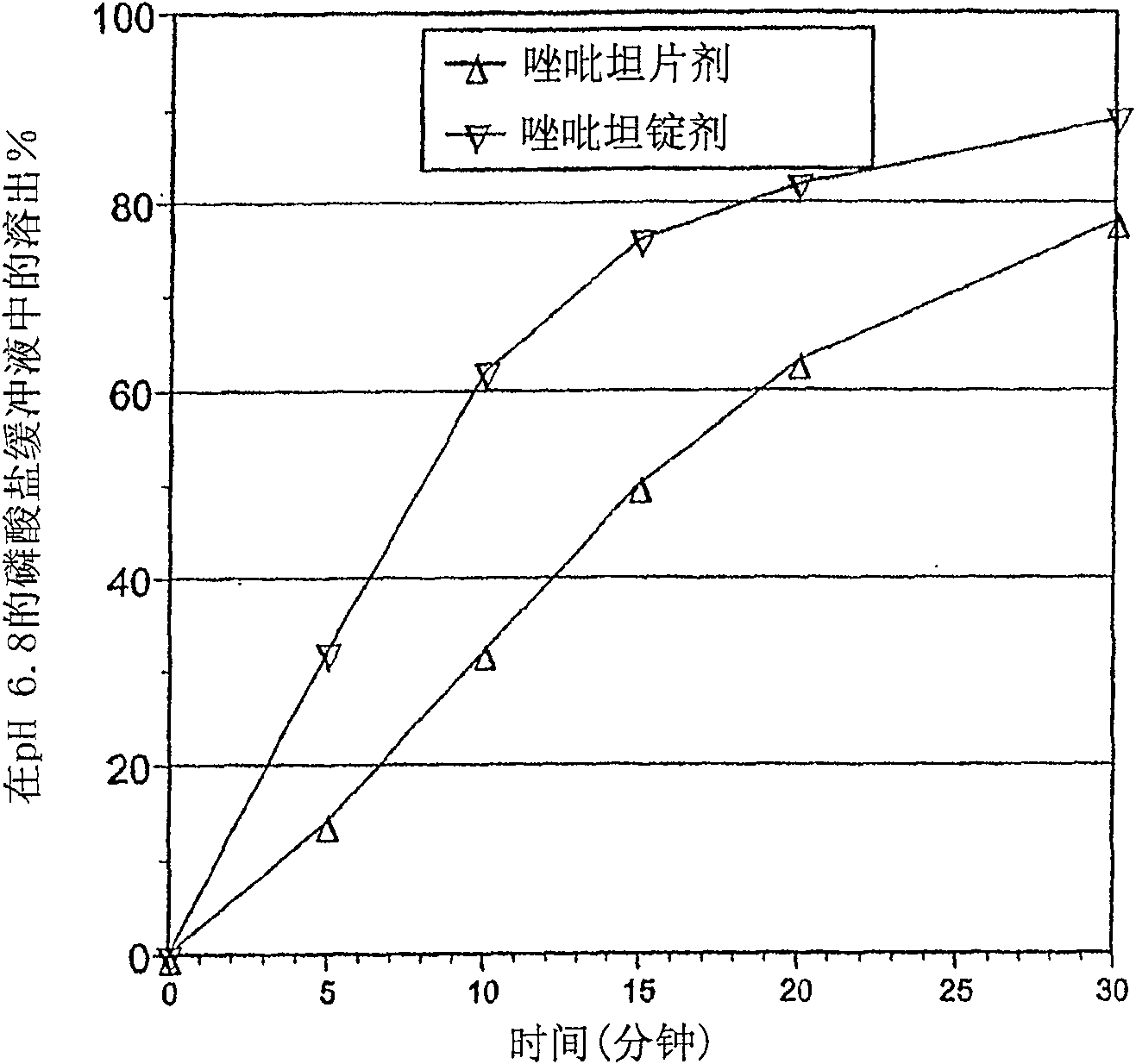
[0249] Embodiment 1. Zolpidem film test
[0250] This example illustrates the beneficial effect of pH adjustment on membrane permeation of zolpidem dosage forms.
[0251] Membrane assays can be used to show the effect of pH adjustment on the degree of ionization and thus on the degree of transmucosal penetration of therapeutic agents, see, e.g., Kansy et al., J. Med. Chem., 41:1007-1010 (1998); and Avdeef, Curr. Topics Med. Chem., 1:277-351 (2001). This test uses lipid-coated membranes to predict membrane penetration of lipidic mucosa. The membrane device consists of a dodecane membrane sandwiched between donor and recipient cells. The lipid-covered membrane is less porous than the oral mucosa. As such, the improvements seen in the membrane assay are likely to be amplified in vivo.
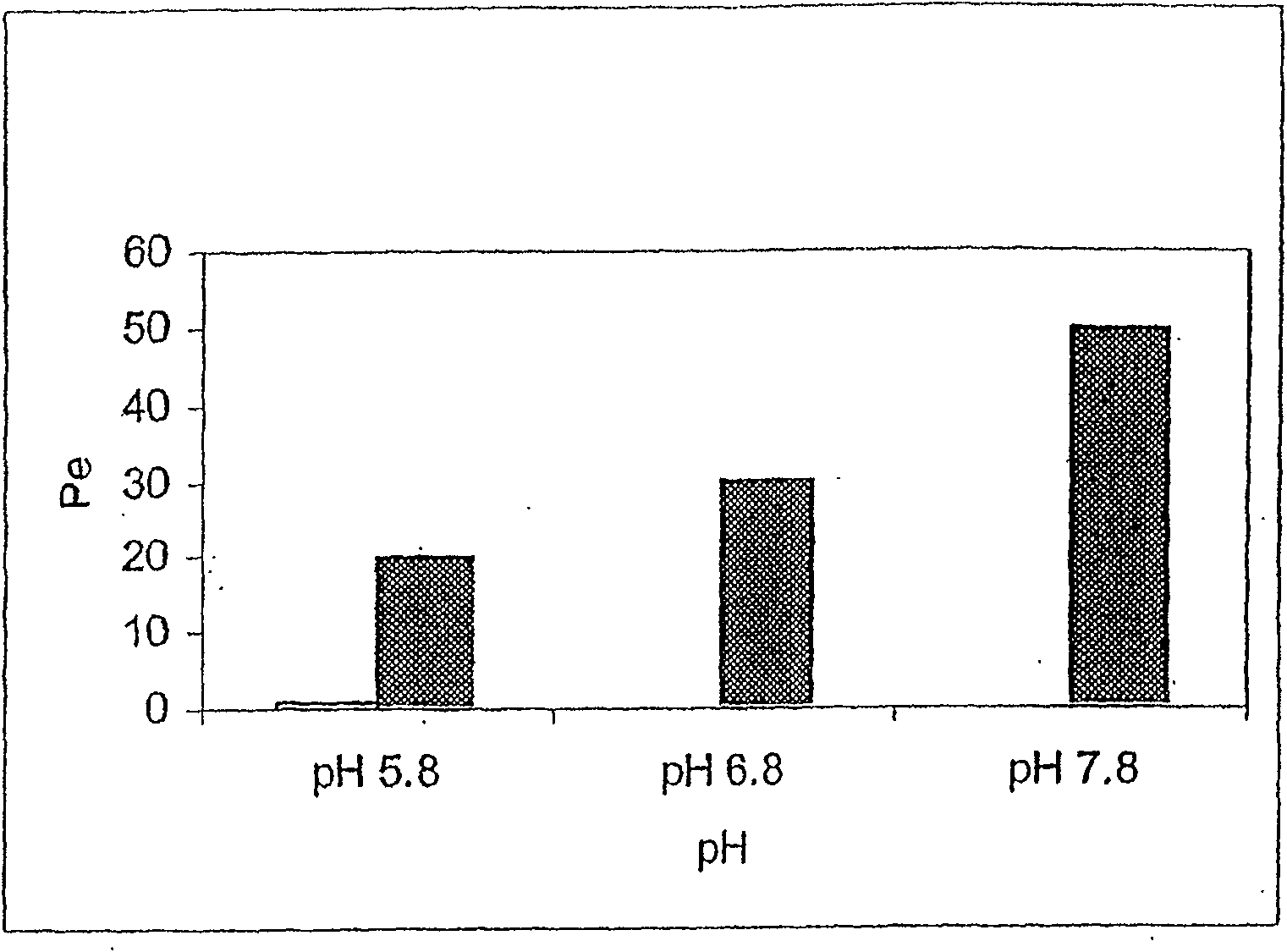
[0252] Membrane tests were performed using zolpidem tartrate solutions at pH conditions of 5.8, 6.8, and 7.8. Adjust the alkaline pH to 7.8 with freshly prepared 0.01 M sodium bicarbonate / sod...
Embodiment 2
[0255] Example 2. Zolpidem Chewing Gum Composition
[0256] This example illustrates the zolpidem chewing gum composition of the present invention.
[0257] Zolpidem can be formulated as a chewing gum composition as described above. In these embodiments, the unit dose or supply of chewing gum comprises from about 0.1 to about 100 milligrams (mg) of zolpidem (measured as its tartrate salt form), preferably from about 1 to about 50 mg, more preferably from about 2 to about 25 mg . In other embodiments, the unit dose comprises from about 2 to about 20 mg of zolpidem, preferably from about 5 to about 15 mg. Additional zolpidem may be added, such as up to about 10% to about 25% by weight, as an "overdose" or as an amount that is expected to be "washed out" and otherwise not released or absorbed during chewing.
[0258] In additional embodiments, the unit dose or supply of chewing gum comprises from about 0.81 to about 42 mg of the base form of zolpidem, more preferably from abou...
Embodiment 3
[0266] Example 3. Zolpidem Tablet Composition
[0267] This example illustrates slow-dissolving, fast-dissolving, and chewable zolpidem tablet compositions of the present invention.
[0268] Zolpidem can be formulated as a tablet composition as described above. In these embodiments, the unit dose or supply of tablets comprises from about 0.1 to about 100 milligrams (mg) of zolpidem (measured as its tartrate salt form), preferably from about 1 to about 50 mg, more preferably from about 2 to about 25 mg . In other embodiments, the unit dose comprises from about 2 to about 20 mg of zolpidem, preferably from about 2 to about 15 mg, more preferably from about 2 to about 10 mg, e.g., about 2, 3, 4, 5, 6, 7, 8 , 9, or 10 mg. In a particularly preferred embodiment, the unit dose comprises an amount of zolpidem that is less than the dose typically used in commercially available oral tablets, but which has comparable or greater bioavailability and efficacy of therapeutic activity. e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com