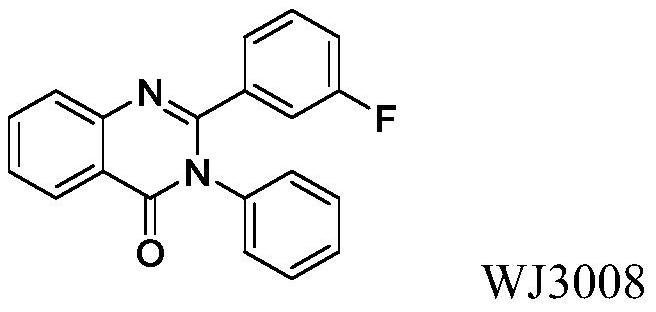
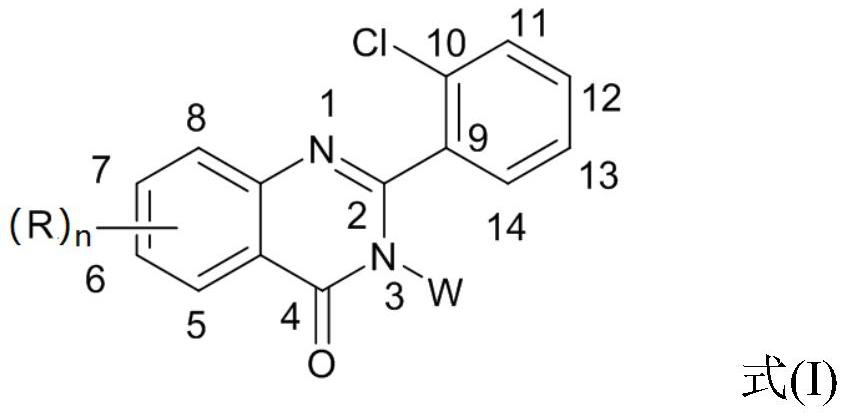
2-(2-Chlorophenyl)quinazolin-4(3h)-one derivatives and their preparation methods and uses
A technology of quinazoline and chlorophenyl, which is applied in the field of pharmaceutical research, can solve problems such as dizziness, cognition and memory impairment, drug daytime sleepiness, abuse, etc., and achieve the reduction of next-day residual effects, strong drug efficacy, and fast metabolism fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
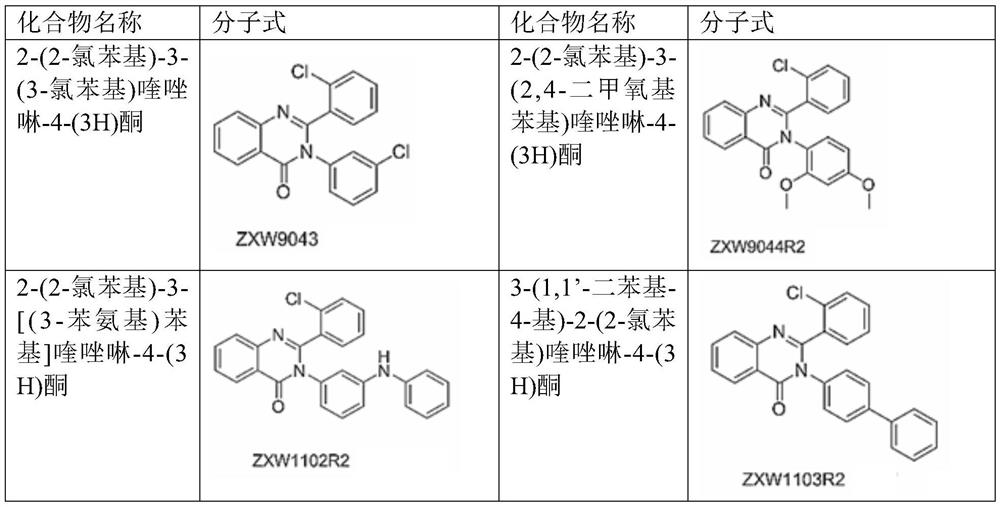
[0069] Example 1, 2-(2-Chlorophenyl)-3-(3-Chlorophenyl)quinazolin-4-(3H)one (Compound No.: ZXW9043)
[0070]
[0071] The first step, the preparation of 2-nitro-N-phenylbenzamide
[0072]
[0073] Weigh 2.0g (0.012mol) of o-nitrobenzoic acid and place it in a 100mL reaction flask, add 7.1g (0.06mol) of thionyl chloride, place the reaction flask in an oil bath, heat to reflux at 80°C, and after 2 hours Terminate the reaction, concentrate under reduced pressure to remove the solvent, add 20 mL of dichloromethane, concentrate again under reduced pressure to remove the solvent, and obtain a light yellow oil, add 20 mL of dichloromethane to form an acid chloride solution for use. Add 1.52g (0.012mol) of 3-chloroaniline to a 100mL reaction flask, add 1.42g (0.018mol) of pyridine, dissolve with 20mL of dichloromethane, add a dropping funnel to the reaction flask, vacuum nitrogen protection, put the reaction flask into In an ice bath, put the acid chloride solution in a droppin...
Embodiment 2
[0080] Example 2 2-(2-chlorophenyl)-3-(2,4-dimethoxyphenyl)quinazolin-4-(3H)one (compound number: ZXW9044R2)
[0081]
[0082] Using 2-amino-N-(2,4-dimethoxyphenyl)benzamide and 2-chlorobenzaldehyde as raw materials, according to the synthesis method described in Example 1, 150 mg of white solid was obtained, yield: 71.4%. 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 8.24-8.21 (dd, J 1 =8.1Hz,J 2 =1.1Hz,1H,ArH),7.94–7.90(m,1H,ArH),7.77(d,J=8.1Hz,1H,ArH),7.69-7.56(m,2H,ArH),7.38-7.10(m ,4H,ArH),6.93-6.82(m,2H,ArH),3.68(s,3H,OCH 3 ),3.61(s,3H,OCH 3 ); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 162.3, 153.3, 148.9, 147.2, 134.9, 134.8, 132.0, 130.6, 130.3, 129.6, 127.8, 127.7, 127.3, 126.6, 121.4, 121.2, 121.2, 120.5, 112.1, 111.0; ESI, m / z) calculated value C 22 h 18 ClN 2 o 3 [(M+H) + ], 393.1000; measured value, 393.0999.
Embodiment 3
[0083] Example 3 2-(2-chlorophenyl)-3-[(3-phenylamino)phenyl]quinazolin-4-(3H)one (compound number: ZXW1102R2)
[0084]
[0085] Using 2-amino-N-(3-(phenylamino)phenyl)benzamide and 2-chlorobenzaldehyde as raw materials, according to the synthesis method described in Example 1, 280 mg of white solid was obtained, yield: 65.9%. 1 H NMR (400MHz, DMSO-d 6 )δ(ppm):8.23(d,J 1 =7.8Hz,1H,ArH),7.95-7.89(m,2H,ArH),7.76(d,J=8.1Hz,1H,ArH),7.65-7.61(m,1H,ArH),7.43-7.31(m ,3H,ArH),6.70-6.67(m,4H,ArH),7.09-6.92(m,4H,ArH),6.70-6.67(m,1H,ArH); 13 C NMR (100MHz, DMSO-d 6 )δ (ppm): 161.9, 153.6, 147.7, 142.4, 141.4, 135.0, 134.8, 131.4, 130.2, 129.7, 129.4, 129.3, 127.9, 127.8, 127.0, 126.8, 122.5, 122.1, 120.3, 119.3; ESI, m / z) calculated value C 26 h 19 ClN 3 O[(M+H) + ], 424.1211; measured value, 424.1211.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com