Ligustrazine stilbenoids derivatives, preparation method thereof, medicament composition and use
A technology of stilbene and derivatives of ligustrazine, which is applied in the field of derivative drugs and can solve the problems of short half-life, accumulated poisoning, and fast metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
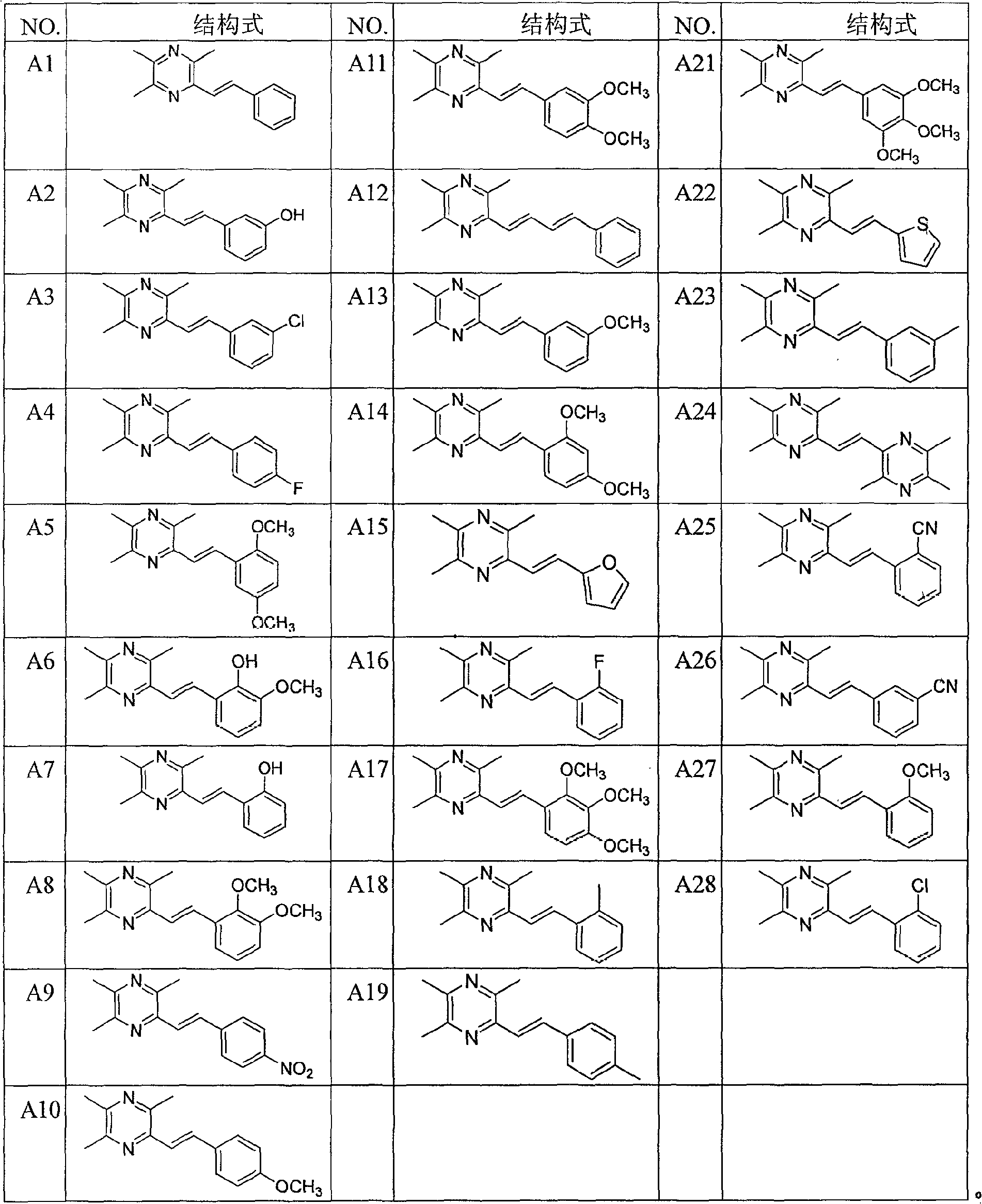
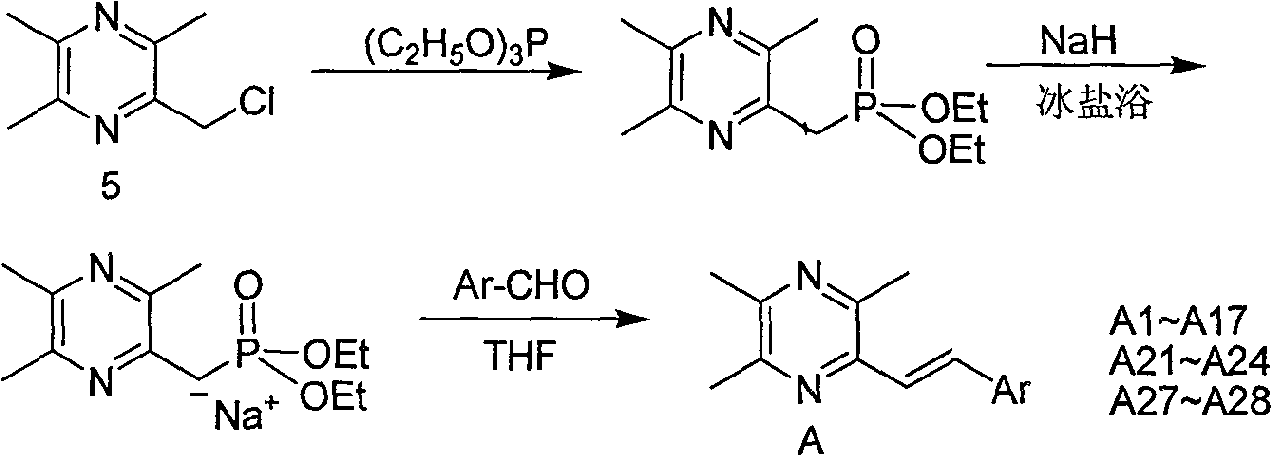
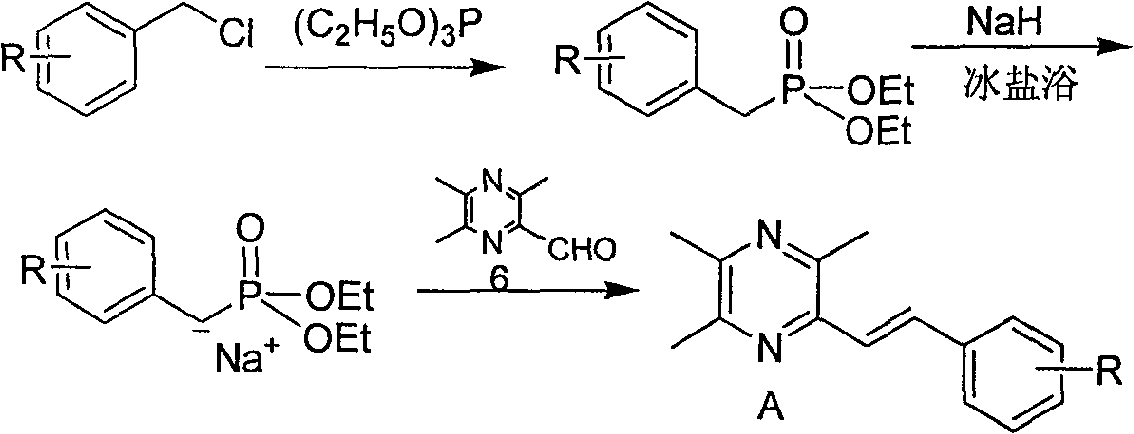
[0052] Example 1: Preparation of 2-styryl-3,5,6-trimethylpyrazine (A1)
[0053] Take 2-chloromethyl-3,5,6-trimethylpyrazine 5 (1.65g, 9.7mmol) in a two-necked flask, add newly distilled triethyl phosphite (1.92ml, 9.7mmol), mix The liquid was heated to slight boiling, and the reaction was monitored by TLC to complete, then cooled to room temperature. Under ice-salt bath conditions (below -5°C), add anhydrous tetrahydrofuran (10ml) and 60% NaH (mass ratio, 0.78g, 19.4mmol), stir well for 40min, then slowly drop into benzaldehyde (1.03g, 9.7mmol ) and anhydrous tetrahydrofuran (25ml) mixed solution, stirred at room temperature for 12h, TLC monitored that the reaction was complete, extracted with ethyl acetate, anhydrous Na 2 SO 4 After drying, the solvent was evaporated, and the residue was separated by flash column chromatography, the eluent was ethyl acetate:cyclohexane (1:10 volume ratio), recrystallized from methanol, and 2-styryl-3,5 was obtained as yellow crystals. , 6-...
Embodiment 2
[0055] Example 2: 2-(3-hydroxystyryl)-3,5,6-trimethylpyrazine (A2)
[0056] The method described in Example 1, except that the mixture of 3-hydroxybenzaldehyde (1.78g, 9.7mmol) and anhydrous tetrahydrofuran (25ml) was slowly added dropwise, and the eluent was ethyl acetate:cyclohexane (1:5 volume ratio) to obtain 0.9 g of white crystals, yield 40%, mp: 202-204 ° C.
[0057] Spectral analysis data: IR(KBr, cm -1 ): 3055 (v =CH ), 1631 (v CH=CH ), 1607, 1578, 1489, 1468 (v CH=CH , Ar), 1407 (v C=N ), 872, 778, 687 (γ =CH outside); 1 H-NMR (DMSO, δppm): 9.49 (1H, s, -OH), 7.59 (1H, d, =CH-, J = 15.6Hz), 7.32 (1H, d, =CH-, J = 15.6Hz) , 7.20 (1H, t, Ar-H, J=7.8Hz), 7.13 (1H, d, Ar-H, J=7.7Hz), 7.10 (1H, s, Ar-H), 6.72 (1H, d, Ar-H, J=7.3Hz), 2.56 (3H, s, -CH 3 ), 2.50 (3H, s, -CH 3 ), 2.44 (3H, s, -CH 3 ); 13 C-NMR (DMSO, δppm): 149.41, 178.67, 146.25, 137.63 (pyrazine-C), 157.54, 137.63, 133.02, 129.62, 122.77, 118.26, 115.51, 113.41 (-CH=CH-Ar-C), 21.31 ( -CH 3 ), ...
Embodiment 3
[0058] Example 3: Preparation of 2-(3-chlorostyryl)-3,5,6-trimethylpyrazine (A3)
[0059] The method described in Example 1, except that the mixture of 3-chlorobenzaldehyde (1.46g, 9.7mmol) and anhydrous tetrahydrofuran (25ml) was slowly added dropwise, and the eluent was ethyl acetate:cyclohexane (1:5 volume ratio) to obtain 0.7 g of yellow crystals, yield 27%, mp: 92-94°C.
[0060] Spectral analysis data: IR(KBr, cm -1 ): 3054 (v =CH ), 1631 (v CH=CH ), 1589, 1559, 1473 (v CH=CH , Ar), 1424, 1405 (v C=N ), 872, 799, 685 (γ =CH out of plane); 1H-NMR (CDCl 3, δppm): 7.73 (1H, d, = CH-, J = 15.6Hz), 7.29 (1H, d, = CH-, J = 15.7Hz), 7.60 (1H, s, Ar-H), 7.47 (1H , d, Ar-H, J=7.6Hz), 7.32 (1H, t, Ar-H, J=7.9Hz), 7.29 (1H, d, Ar-H, J=7.4Hz), 2.64 (3H, s ,-CH 3 ), 2.56 (3H, s, -CH 3 ), 2.54 (3H, s, -CH 3 ); 13 C-NMR (CDCl 3 , δppm): 150.62, 149.27, 147.29, 144.85 (pyrazine-C), 138.91, 134.72, 132.52, 129.93, 128.19, 126.84, 1125.50, 124.39 (-CH=CH-Ar-C); 21.78, 20.96 (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com