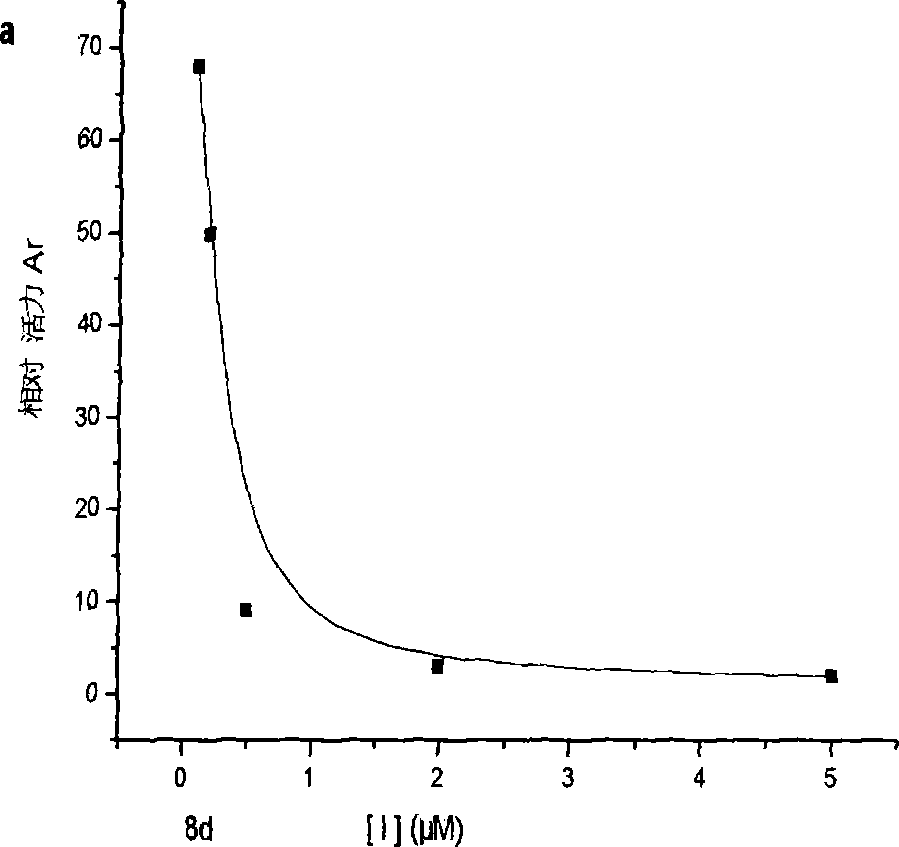
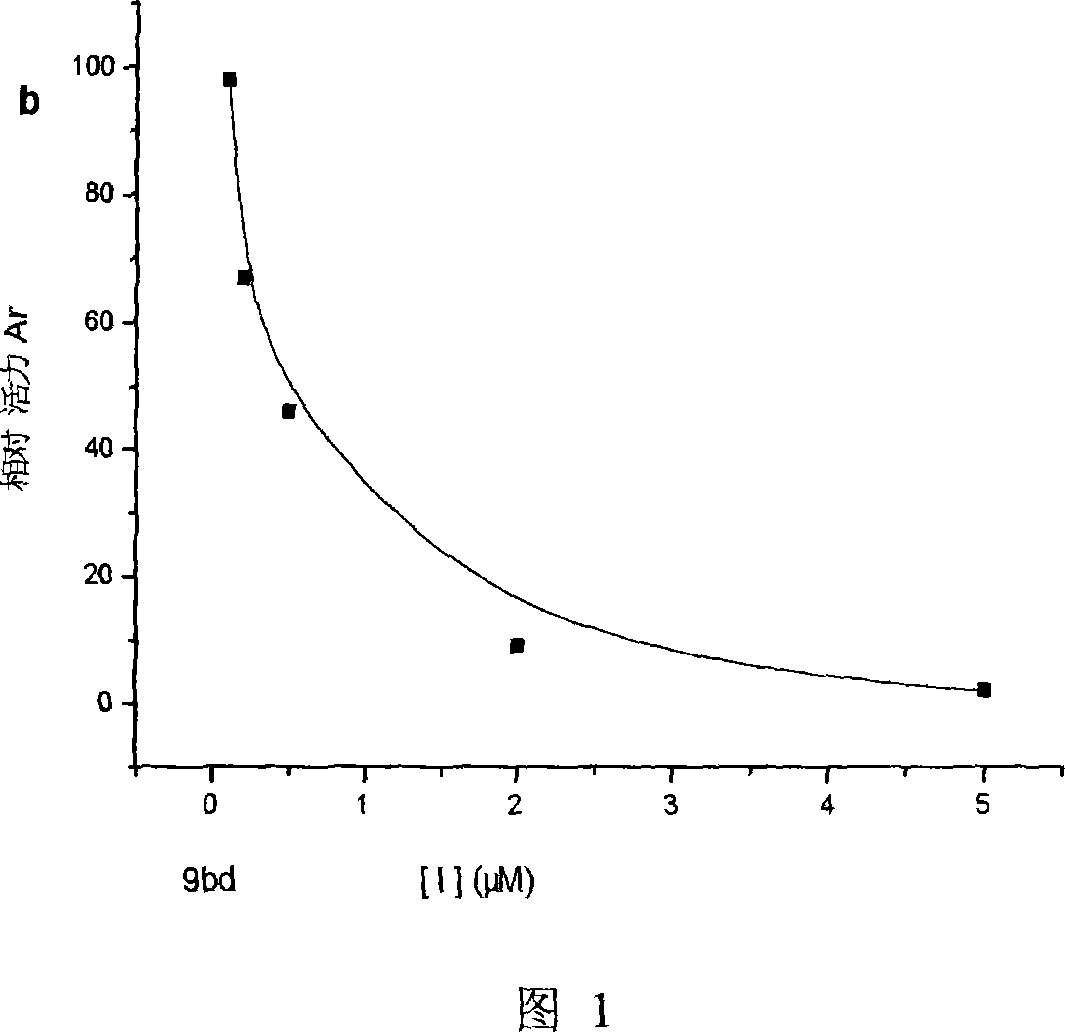
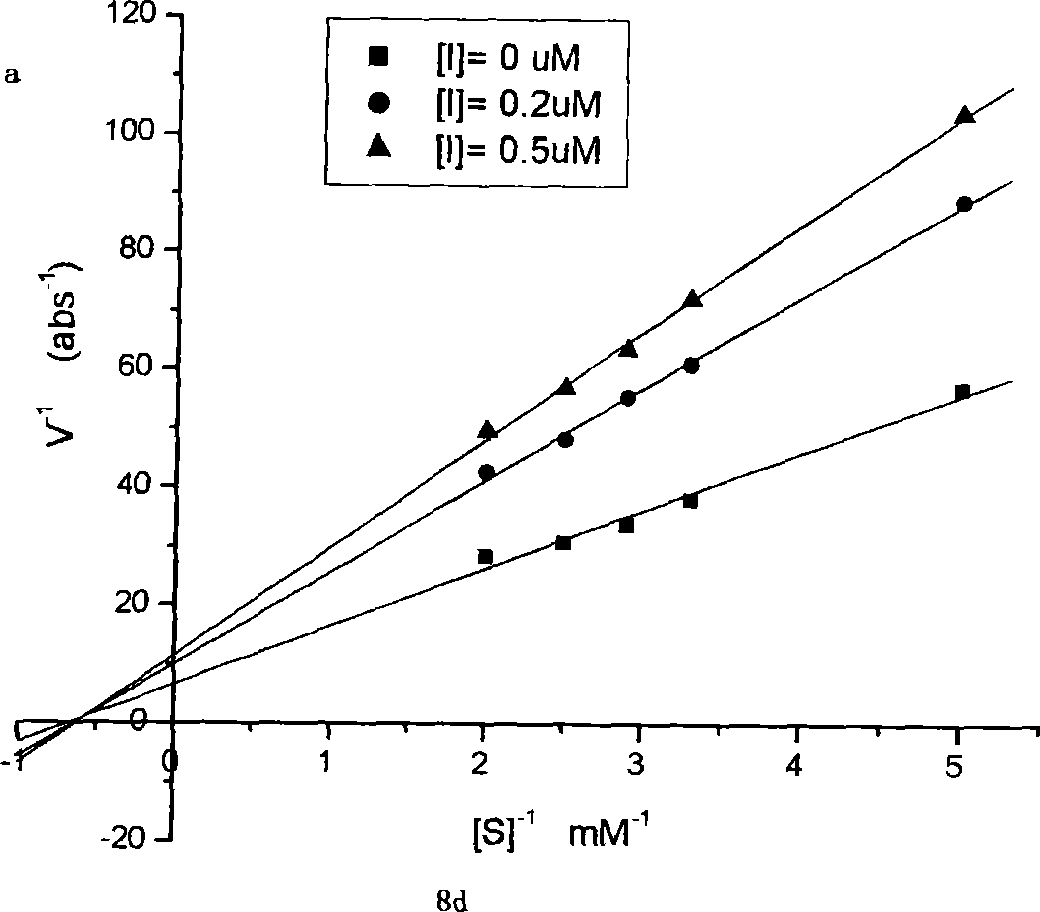
Bean-curd pectin analogues, and its use in preparation of medicine for preventing senile dementia
A technology for senile dementia and tofu glucoside, which is applied in the direction of drug combination, sugar derivatives, pharmaceutical formulas, etc., can solve the problem of no structure modification of tofu glucoside, and achieve the effect of low toxic and side effects and high curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation of the tofu fruit glycoside analog of the present invention is shown in the following reaction formula 1-2:
[0045] Reaction 1:
[0046]
[0047] 2 1 , R 2 , R 3 , R 4 =Ac 3a R 1 , R 2 , R 3 , R 4 = H R 5 =NHCSNH 2
[0048] 2b 1 , R 2 , R 3 , R 4 =Bz 3b R 1 , R 2 , R 3 , R 4 = H, R 5 =NHCONH 2
[0049] 2c 1 , R 2 , R 3 , R 4 =Bn 3c R 1 , R 2 , R 3 , R 4 = H, R 5 =OH
[0050] 2d R 1 , R 2 =C(CH 3 ) 2 ; 3 , R 4 =H 3d R 1 , R 2 , R 3 , R 4 = H, R 5 =OCH 3
[0051] 2 1 , R 2 = H; R 3 , R 4 =PhCH 3ab R 1 , R 2 , R 3 , R 4 =Ac,R 5 =NHCONH 2
[0052] 2F 4 =PO(OCH 3 ) 2 ; 1 , R 2 , R 3 =H 3ac R 1 , R 2 , R 3 , R 4 =Ac,R 5 =OH
[0053] 2g R 4 =C(Ph) 3 ; 1 , R 2 , R 3 =H 3ad R 1 , R 2 , R 3 , R 4 =Ac,R 5 =OCH 3
[0054] 2h 4 = H; R 1 , R 2 , R 3 =Ac 3ba R 1 , R 2 , R 3 , R 4 =Bz,R 5 =NHCSNH 2
[0055] 3bb R 1 , R 2 , R 3 , R ...
Embodiment 1
[0067] Example 1: Synthesis of 2,3,4,6-O-tetraacetyl tofu glucoside (2a)
[0068] First add 1.0g tofu glucoside (1, 3.5mmol) and 2mL anhydrous pyridine into a round bottom flask, stir until completely dissolved, then add 2.5g acetic anhydride (25mmol) dropwise, and react at room temperature for 12h. After pouring into 50 ml of ice water and stirring for half an hour, the chloroform layer was separated and extracted. After several times of washing with ice water, drying with anhydrous magnesium sulfate and rotary evaporation to dry the solvent, a light yellow syrupy substance was obtained, which was recrystallized from 95% ethanol to obtain white solid powder 2a with a yield of 56.1%. The melting point is 135-136°C. The structure of the product was determined by IR, NMR, MS and elemental analysis data.
[0069] Anal. Calcd for C 21 h 24 o 11 : C55.75, H5.35; found: C55.79, H5.26; IR (KBr, cm -1 )υ: 1752(C-O), 1693(CHO), 1601(Ph), 1506(Ph), 1224(C-O), 1157(C-O-C), 1127(C-O...
Embodiment 2
[0070] Example 2: Synthesis of 2,3,4,6-O-tetrabenzoyl curcurin (2b)
[0071] First, 1.0 g tofu glucoside (1, 3.5 mmol) was dissolved in 20 mL of chloroform. Under stirring at room temperature, 3.5 g of benzoyl chloride (25 mmol) and 4 mL of anhydrous pyridine were successively added. Then react at room temperature for 24h. 10 mL of methanol was added to terminate the reaction, and after stirring for 10 minutes, the solvent was spun to dryness to obtain an oily substance. Add chloroform to dilute, wash with dilute hydrochloric acid, saturated sodium bicarbonate solution, and ice water, respectively. After concentration under reduced pressure, white solid powder 2b was obtained by silica gel column chromatography with a yield of 47.7%. The melting point is 182.9-183.2°C. The structure of the product was determined by IR, NMR, MS and elemental analysis data.
[0072] Anal. Calcd for C 41 h 32 o 11 : C70.28, H4.60, found: C70.37, H4.49; IR (KBr, cm -1 )υ: 1752(C-O), 1690(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com