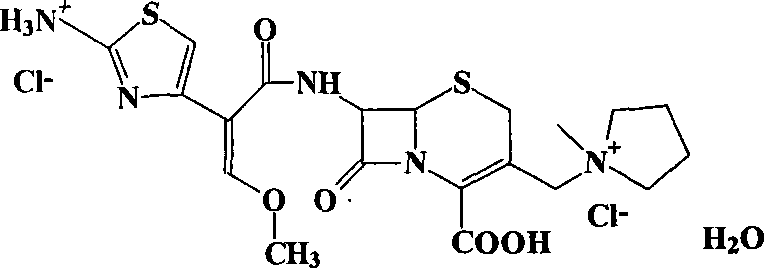
Preparation of cefepime halogen acid salt by calcium salt precipitation process
A technology of hydrohalide salt and cefepime, which is applied in the field of preparation of cephalosporins, can solve problems such as pollution, and achieve the effects of reducing emissions, saving production costs and prolonging service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]Dissolve 10.5g of calcium chloride dihydrate (71mmol, 0.9 times the molar ratio of cefepime sulfate) in 110mL of water at room temperature, and then add 40g of cefepime sulfate (79mmol, weight based on anhydrous matter) in batches under effective stirring. ), forming a slurry system with good fluidity. After 10 minutes, add 180 mL of acetone and appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with an appropriate amount of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. 1200 mL of acetone was added to the filtrate under stirring, and the addition was completed within about 1 hour. The temperature of the system was lowered to 0-5°C again, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40°C to obtain about 35.2 g of white crystalline powder. The water content is 3.7% (K-F method),...
Embodiment 2
[0035] Dissolve 12.8g of calcium chloride dihydrate (87mmol, 1.1 times the molar ratio of cefepime sulfate) in 110mL of water at room temperature, and then add 40g of cefepime sulfate (79mmol, weight based on anhydrous matter) in batches under effective stirring. ), forming a slurry system with good fluidity. After 10 minutes, add 180 mL of acetone and appropriate amount of activated carbon, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with an appropriate amount of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. 1200 mL of acetone was added to the filtrate under stirring, and the addition was completed within about 1 hour. The temperature of the system was lowered to 0-5°C again, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 36 g of white crystalline powder. The product is cefepime dihydrochlorid...
Embodiment 3
[0037] Dissolve 11.6g of calcium chloride dihydrate (79mmol, 1.0 times the molar ratio of cefepime sulfate) in 90mL of water and 20mL of methanol at normal temperature, then add 40g of cefepime sulfate (79mmol, weight according to water content), forming a slurry system with good fluidity. After 10 minutes, 90 mL of methanol and an appropriate amount of activated carbon were added, and stirred for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with an appropriate amount of acetone-water 2:1 (v / v) mixed solution, and combine the filtrates. 1500 mL of acetone was added to the filtrate under stirring, and the addition was completed within about 1 hour. The temperature of the system was lowered to 0-5°C again, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40° C. to obtain about 34.8 g of white crystalline powder. The product is cefepime dihydrochloride mon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com