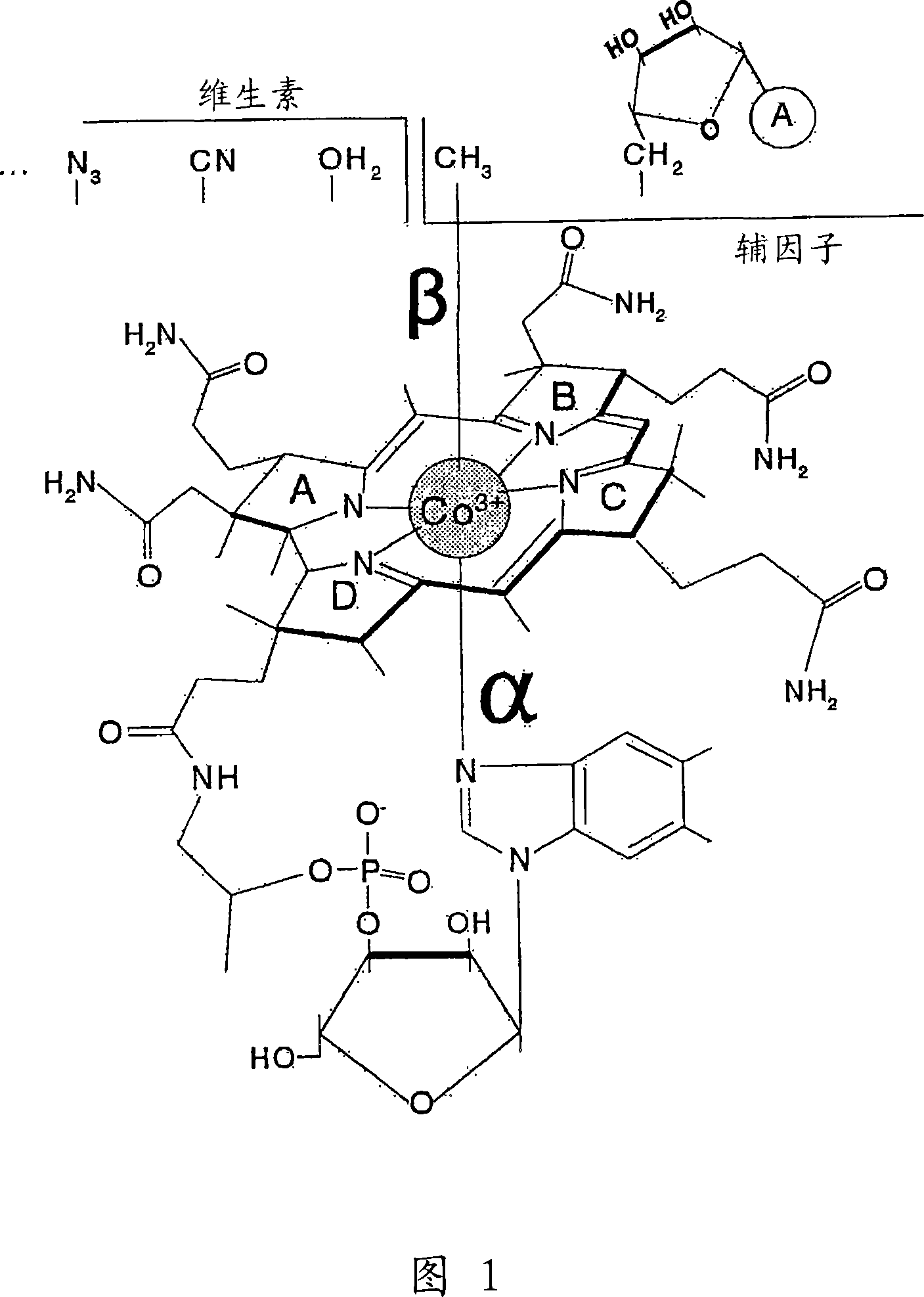
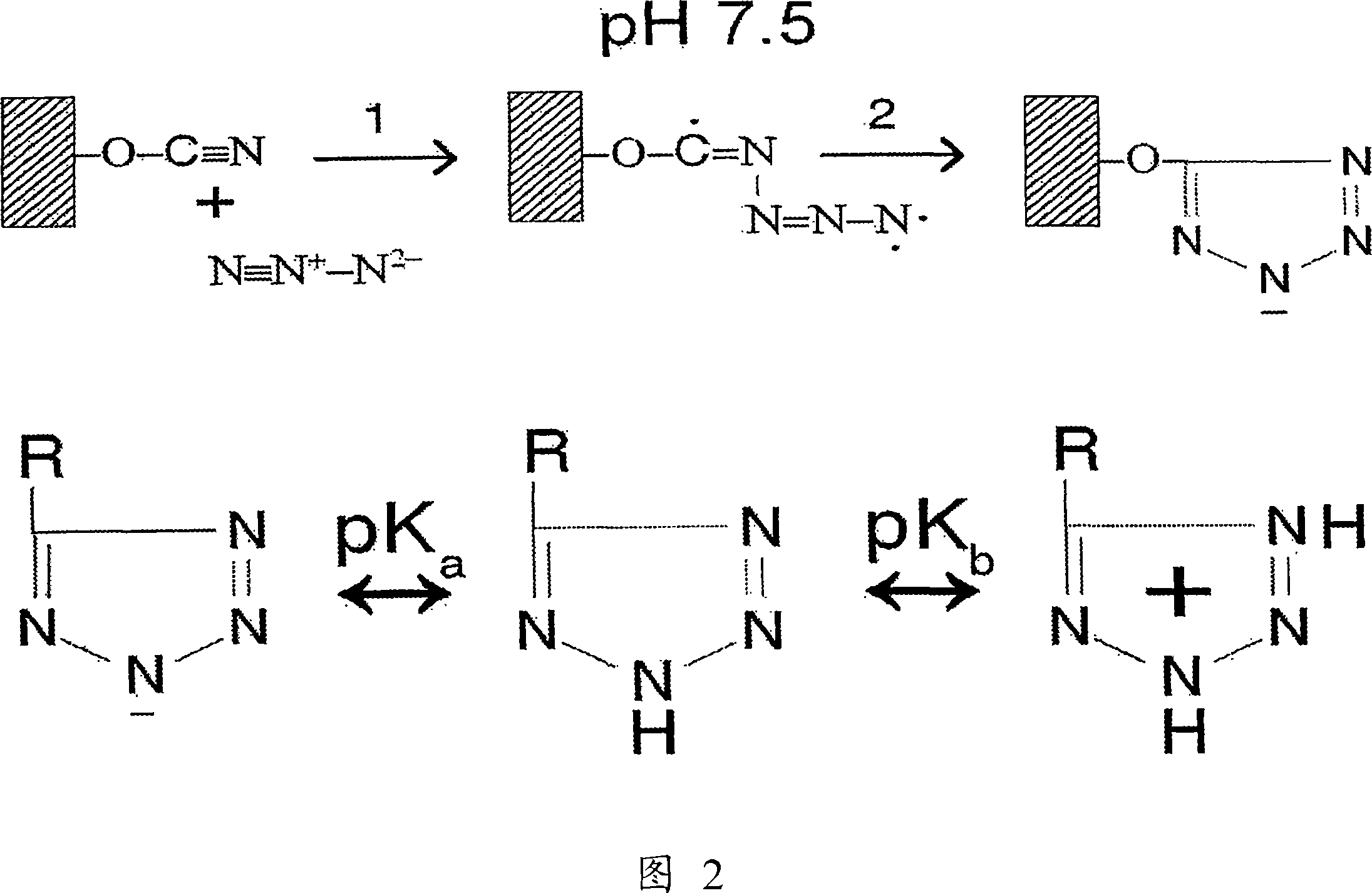
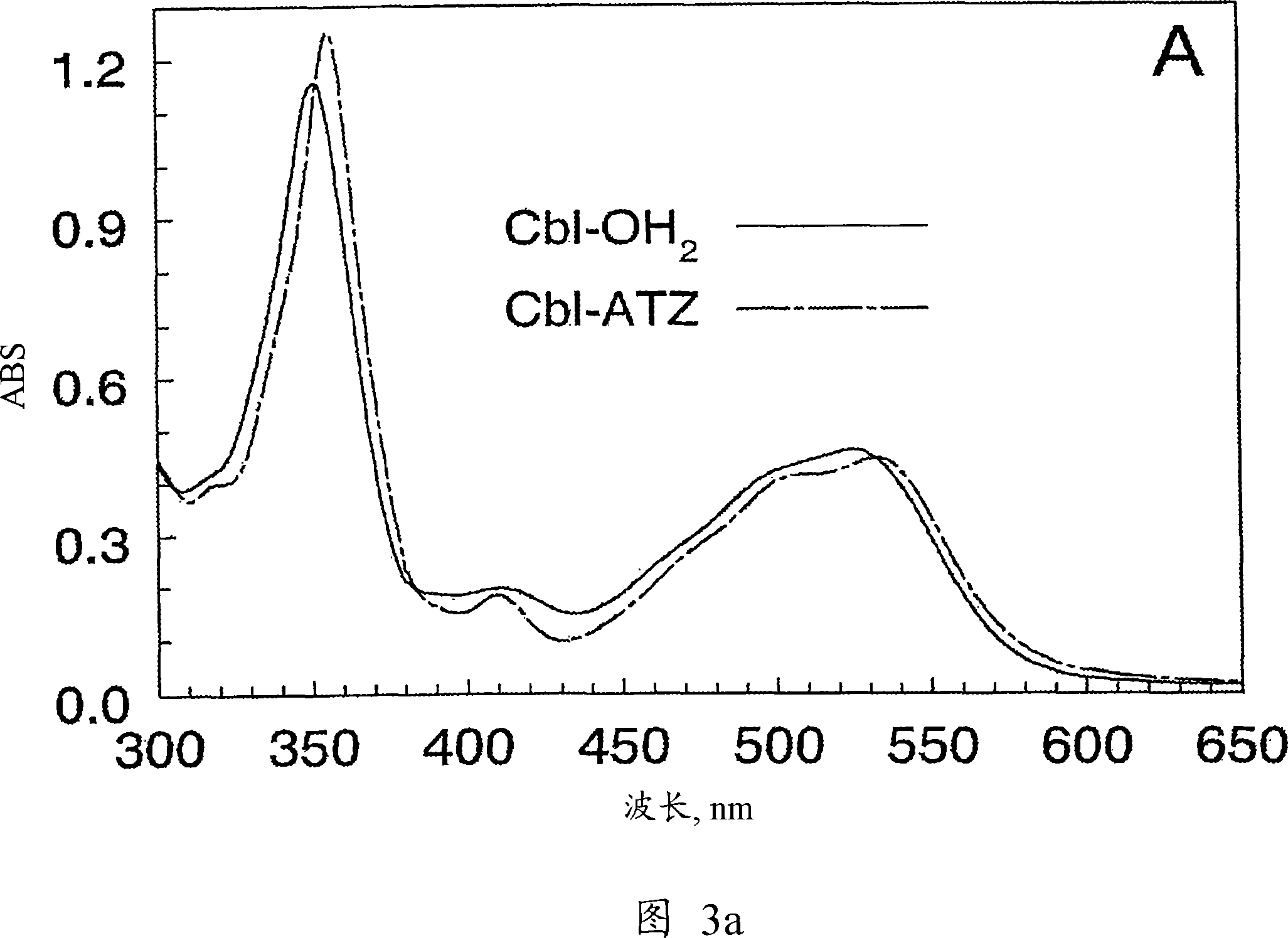
Methods for purifying corrin-containing molecules
A corrin and molecular technology, applied in the field of preparing this kind of adsorbent material, can solve the problems that cannot be used to purify vitamins, slowness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] 1.1 Preparation of absorbent material
[0101] CNBr-activated Sepharose 4B (Pharmacia) was loaded onto the column, washed extensively with 1 mM HCl, followed by washing with 0.02M NaN 3 in 0.1M NaH 2 PO 4 Buffer (P i -buffer) at pH 7.5 at room temperature. Within 1 hour, two bed volumes of media were pumped through the column.
[0102] When 1 volume of acid-washed matrix and 2-3 volumes of 0.02M NaN 3 at 0.2MP i - The solution in buffer, pH 7.5 was incubated for 1 hour with regular agitation, and the reaction between agarose and azide could also be performed in batches. After the coupling step, the absorbent material was washed with 0.1M P i - Buffer, pH 6.0-7.0 wash followed by water. The prepared material was stored at room temperature in a suspended absorbent material:water (1:1) ratio.
[0103] 1.2 Chemistry of the reaction
[0104] The exact reaction mechanism between CNBr-activated agarose and azide is unknown. According to the relevant data in literatu...
Embodiment 2
[0107] Example 2. CbI-OH 2 Properties of Specific Adsorbents
[0108] 2.1. Batch saturation of the azide-activated substrate with CbI to the greatest possible extent
[0109] CbI-OH was tested 2 Binding to prepared sorbent material. When 0.05ml of suspended adsorbent material: water (1:1) and 0.5ml of 1mM CbI-OH 2 0.1M P at pH 6 i - Buffer, mixed and incubated for 5 minutes to observe sufficient saturation of the adsorbent material with CbI. The sample was centrifuged (10,000rpm, 30s), and 2ml of 0.1M P i - buffer, wash away the colored supernatant for 1 min. The sorbent material was pelleted by centrifugation and bound CbI was eluted from the pellets as described in Section 2.2. The concentration of eluted CbI was detected according to its absorption spectrum. The concentration of CbI-binding groups in the loaded sorbent was estimated to be 4-8 mM. The amount of reactive groups in the resin depends on the original agarose. Fresh CNBr-activated agarose showed high le...
Embodiment 3
[0138] Example 3. Model purification of CbI
[0139] 3.1. Source of CbI
[0140] Using CbI-OH 2 The model system was established by saturating Cbl-binding intrinsic factor (IF) (5, 12) and adding to human plasma diluted 1:5 with 0.1 M citrate buffer pH 3.0. The final concentration of IF-CbI was 10 μM (≈0.01 mg / ml of CbI) in a total volume of 5 ml. The concentration of contaminating protein was about 15 mg / ml.
[0141] 3.2. Extraction of CbI
[0142] Heat the CbI-containing sample at 95 °C for 30 min to release the CbI-OH from the protein complex 2 . Treatment at pH 3 was accompanied by about 30% ligand degradation. For comparison, a similar treatment at pH 7 resulted in CbI-OH 2 complete degradation, which may be due to the reduction of SH-containing compounds present in plasma. The heated sample was cooled and neutralized to pH 5.0 with NaOH and debris was removed by centrifugation. A pellet corresponding to 1 / 3 of the total volume was then washed with 0.1 M citrate ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap