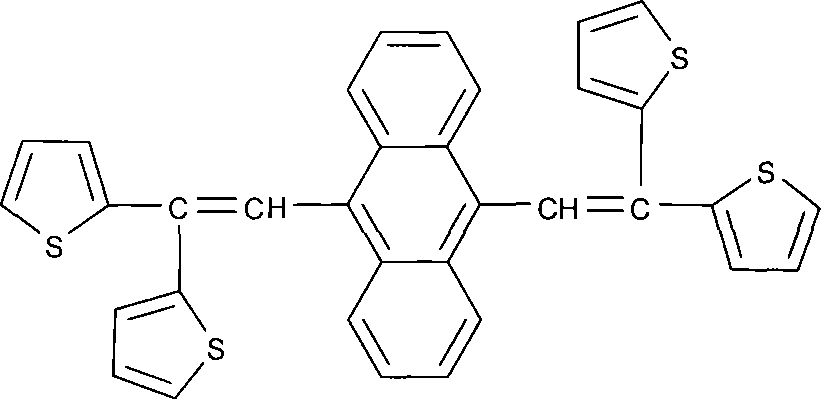
Bi-vinyl anthracenes luminescent compounds
A technology of light-emitting compounds, divinyl anthracene, applied in the fields of light-emitting materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of few types of light-emitting compounds, and achieve the effect of good thermal stability and high melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
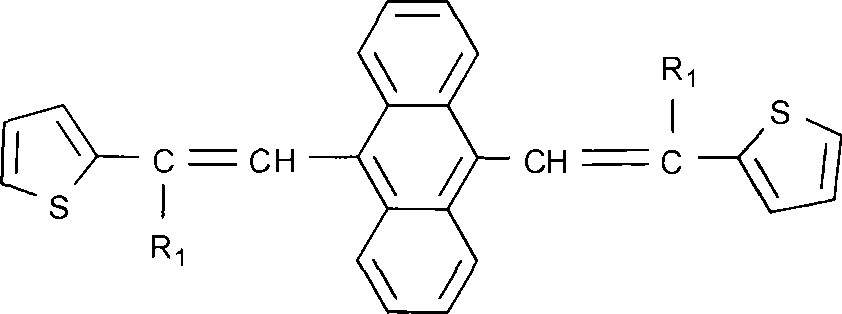
[0025] Embodiment 1: 9, the synthesis of 10-bis[2,2-bis(2-thiophene) base vinyl] anthracene
[0026] 1.1 The present invention is implemented with reference to the following synthetic route
[0027]
[0028] 1.2 Synthesis of 9,10-bis[2,2-bis(2-thienylvinyl]anthracene
[0029] 1.2.1 Synthesis of raw materials
[0030] The 9,10-bis(diethoxyphosphorylmethyl)anthracene used in the present invention is synthesized according to the method disclosed in the literature [Functional Materials, 2004, 5(35):595-597]; Synthesized by the method disclosed in [Organic syntheses Coll. Vol.2, 520; Vol.12, 62].
[0031] 1.2.2 Synthesis of products
[0032] In a 250ml four-neck flask, add 9.8g of 9,10-bis(diethoxyphosphorylmethyl)anthracene, 5.1g of sodium hydride, 8.5g of bis(2-thiophene) ketone, and 100ml of tetrahydrofuran, and start stirring. Heat to reflux. After 4 hours of reflux reaction, the reaction was stopped, and the mixture was naturally cooled to room temperature under stirri...
Embodiment 2
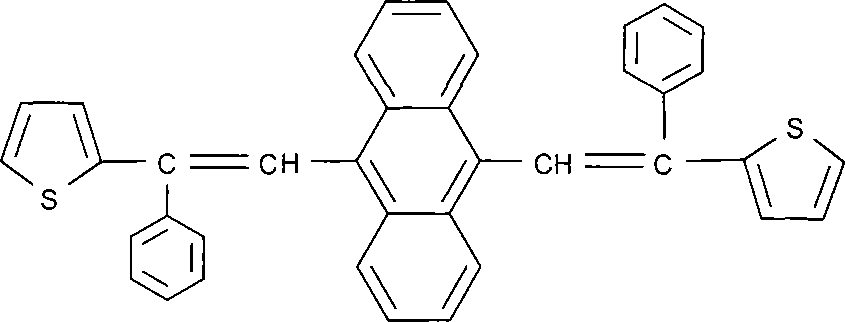
[0044] Example 2: Synthesis of 9,10-bis[2-(2-thiophene)-2-phenylvinyl]anthracene
[0045] 2.1 The present invention is implemented with reference to the following synthetic route
[0046]
[0047] 2.2 Synthesis of 9,10-bis[2-(2-thiophene)yl-2-phenylethenyl]anthracene
[0048] 2.2.1 Synthesis of raw materials
[0049] 9,10-bis(diethoxyphosphorylidene)anthracene used in the present invention is synthesized with reference to the method disclosed in the literature [Functional Materials, 2004, 5(35):595-597]; thienylbenzophenone is referred to Synthesized by the method disclosed in the literature [Organic syntheses Coll. Vol.2, 520; Vol.12, 62].
[0050] 2.2.2 Synthesis of products
[0051] In a 250ml four-necked flask, add 9g of 9,10-bis(diethoxyphosphormethylene)anthracene, 5g of sodium hydride, 8.3g of 2-thienylbenzophenone, and 100ml of tetrahydrofuran, start stirring, and heat up to Reflux, after reflux reaction for 4 hours, stop the reaction, naturally cool to room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com