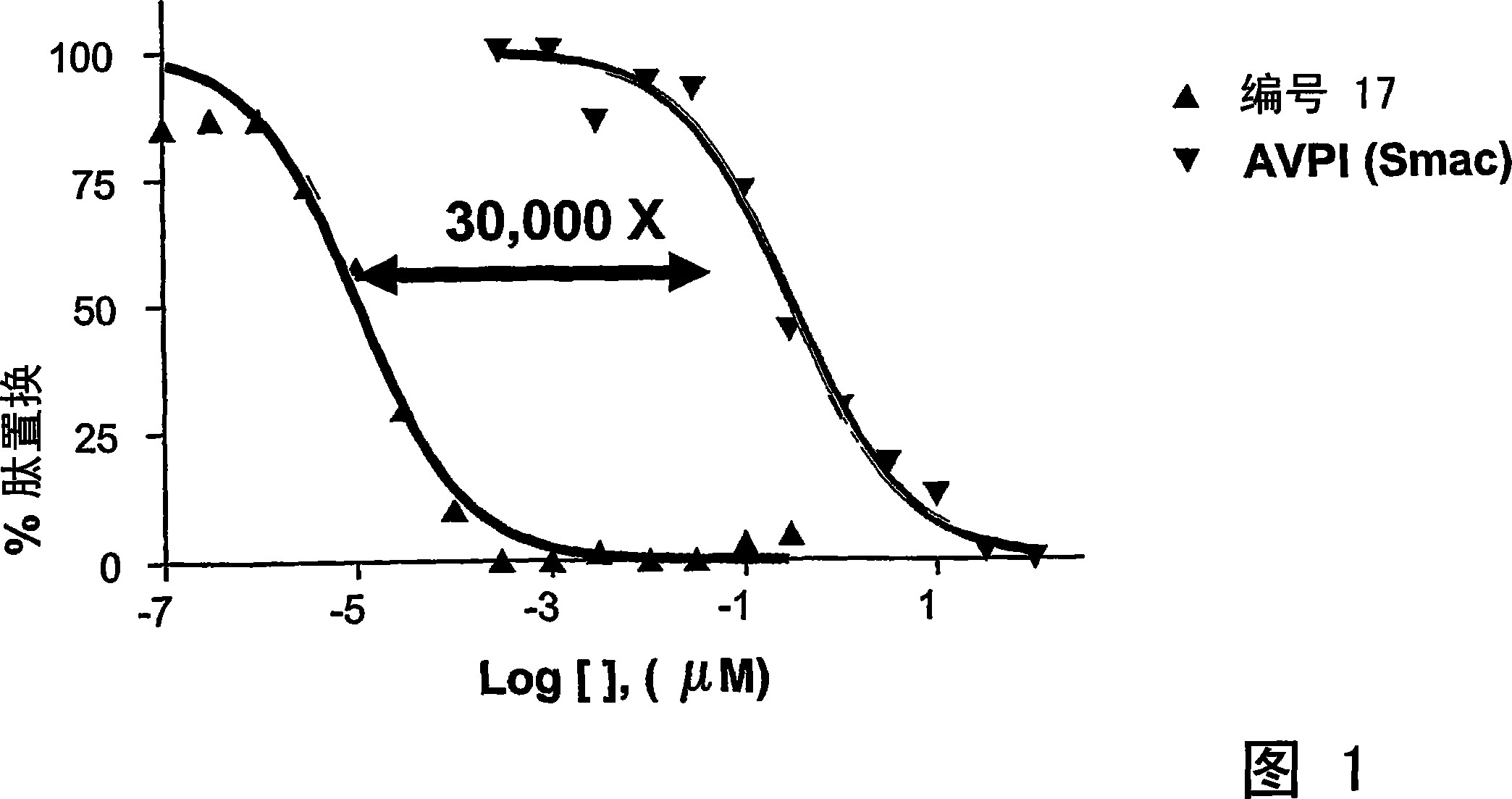
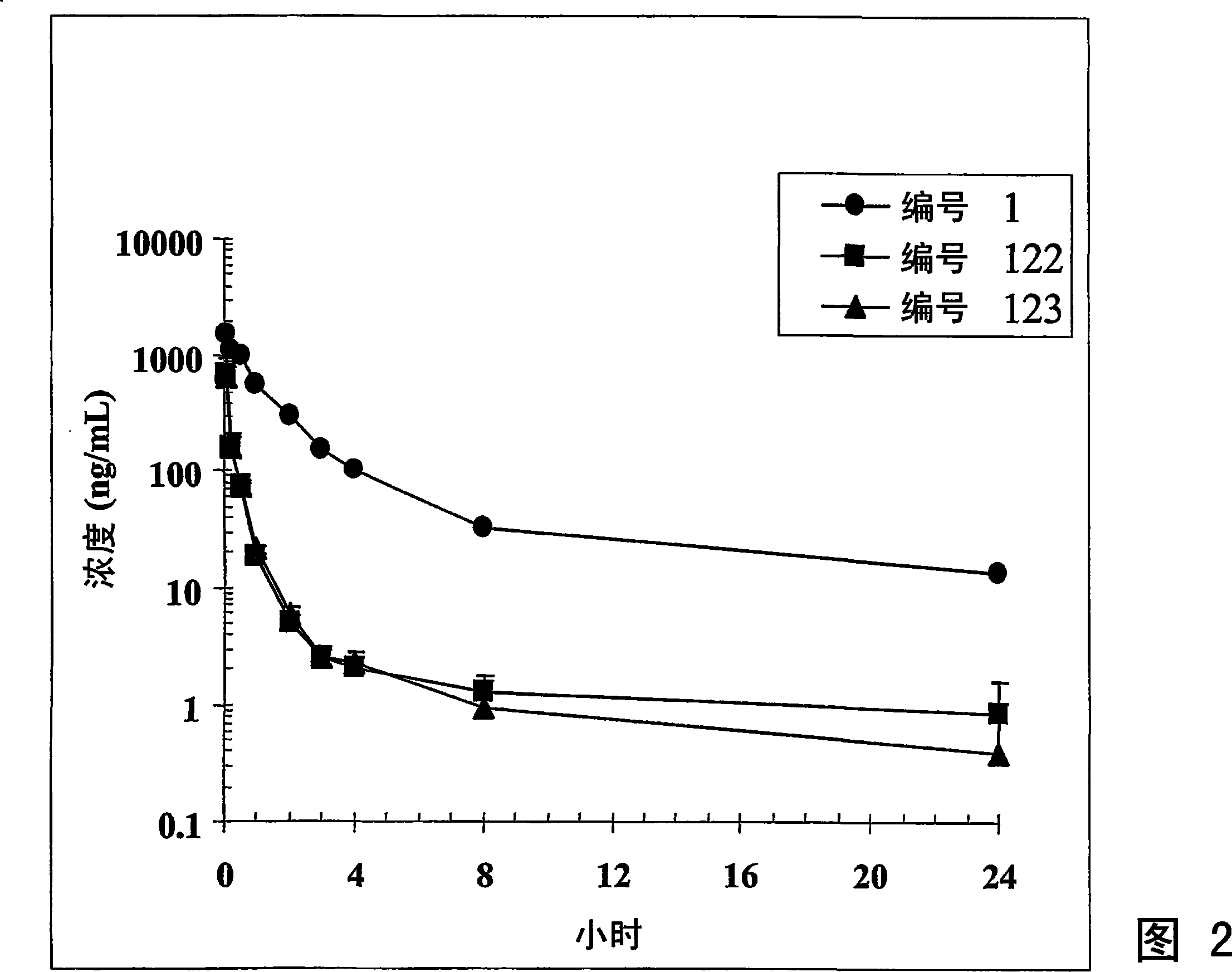
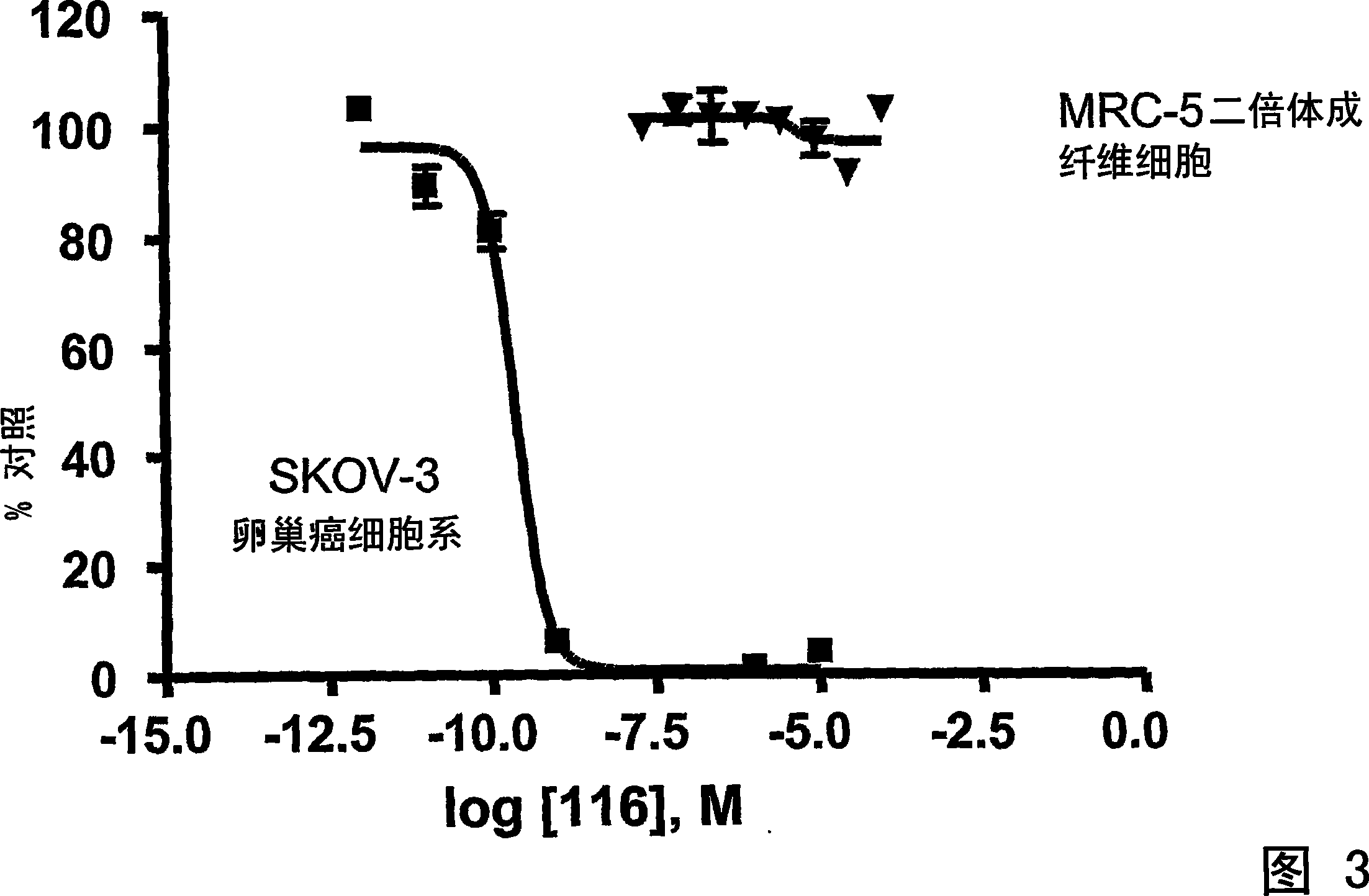
Dimeric IAP inhibitors
An alkyl-independent technology for use in the field of IAP dimer inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Wherein R7a and R7b are independently H, alkyl, cycloalkyl, haloalkyl; or R8a and R7a and R8b and R7b can independently or together form a ring, such as aziridine or azetidine ring;
[0130] R8a and R8b are independently H, hydroxyl, alkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, heteroaryl or heteroaralkyl, wherein alkyl, aryl, aralkyl, Cycloalkyl, cycloalkylalkyl, heteroaryl, and heteroaralkyl are optionally substituted by halogen, hydroxy, mercapto, carboxy, alkyl, alkoxy, amino, and nitro; or R8a And R7a and R8b and R7b can form a ring independently or together, such as aziridine or azetidine ring;
[0131] Wherein R5a and R5b are independently H, alkyl, cycloalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, aralkyl, heteroaryl, heteroaralkyl; or each use the following groups Optionally substituted with: hydroxy, mercapto, halo, amino, carboxyl, alkyl, haloalkyl, alkoxy, or alkylthio; or, in some cases, the R5a and R5b residues are linked via: 2 - alkylene, alke...
Embodiment 2
[0143] Wherein R7a and R7b are independently H, alkyl, cycloalkyl, haloalkyl; or R8a and R7a and R8b and R7b can independently or together form a ring, such as aziridine or azetidine ring;
[0144] R8a and R8b are independently H, hydroxyl, alkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, heteroaryl or heteroarylalkyl, wherein alkyl, aryl, aralkyl , cycloalkyl, cycloalkylalkyl, heteroaryl, and heteroarylalkyl are optionally substituted by halogen, hydroxy, mercapto, carboxy, alkyl, alkoxy, amino, and nitro; Or R8a and R7a and R8b and R7b can form a ring independently or together, such as aziridine or azetidine ring;
[0145] Wherein R5a and R5b are independently H, alkyl, cycloalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, aralkyl, heteroaryl, heteroarylalkyl; or each use the following groups Optionally substituted with: hydroxy, mercapto, halo, amino, carboxy, alkyl, haloalkyl, alkoxy, or alkylthio; or, in some cases, the R5a and R5b residues are linked via: Alkylene, al...
Embodiment 3
[0155] Wherein R7a and R7b are independently H, alkyl, cycloalkyl, haloalkyl; or R8a and R7a and R8b and R7b can independently or together form a ring, such as aziridine or azetidine ring;
[0156] R8a and R8b are independently H, hydroxyl, alkyl, aryl, aralkyl, cycloalkyl, cycloalkylalkyl, heteroaryl or heteroarylalkyl, wherein alkyl, aryl, aralkyl , cycloalkyl, cycloalkylalkyl, heteroaryl, and heteroarylalkyl are each optionally substituted by halogen, hydroxy, mercapto, carboxy, alkyl, alkoxy, amino, and nitrate or R8a and R7a and R8b and R7b can form a ring independently or together, such as aziridine or azetidine ring;
[0157] R5a and R5b are independently H, alkyl, cycloalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, aralkyl, heteroaryl, heteroarylalkyl; or each uses the following groups Optionally substituted with: hydroxy, mercapto, halo, amino, carboxyl, alkyl, haloalkyl, alkoxy, or alkylthio; or, in some cases, the R5a and R5b residues are linked via: 2 - alkylene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com