Organic acid menthol derivative and transdermal drug delivery preparation having the same
A menthol enanthate and drug delivery technology, which is applied in the field of menthol ester derivatives, can solve the problems of single drug, unimproved volatility, production and storage barriers of percutaneous absorption preparations, etc., and achieve wide application prospects, Enhanced through ability, easy to save the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
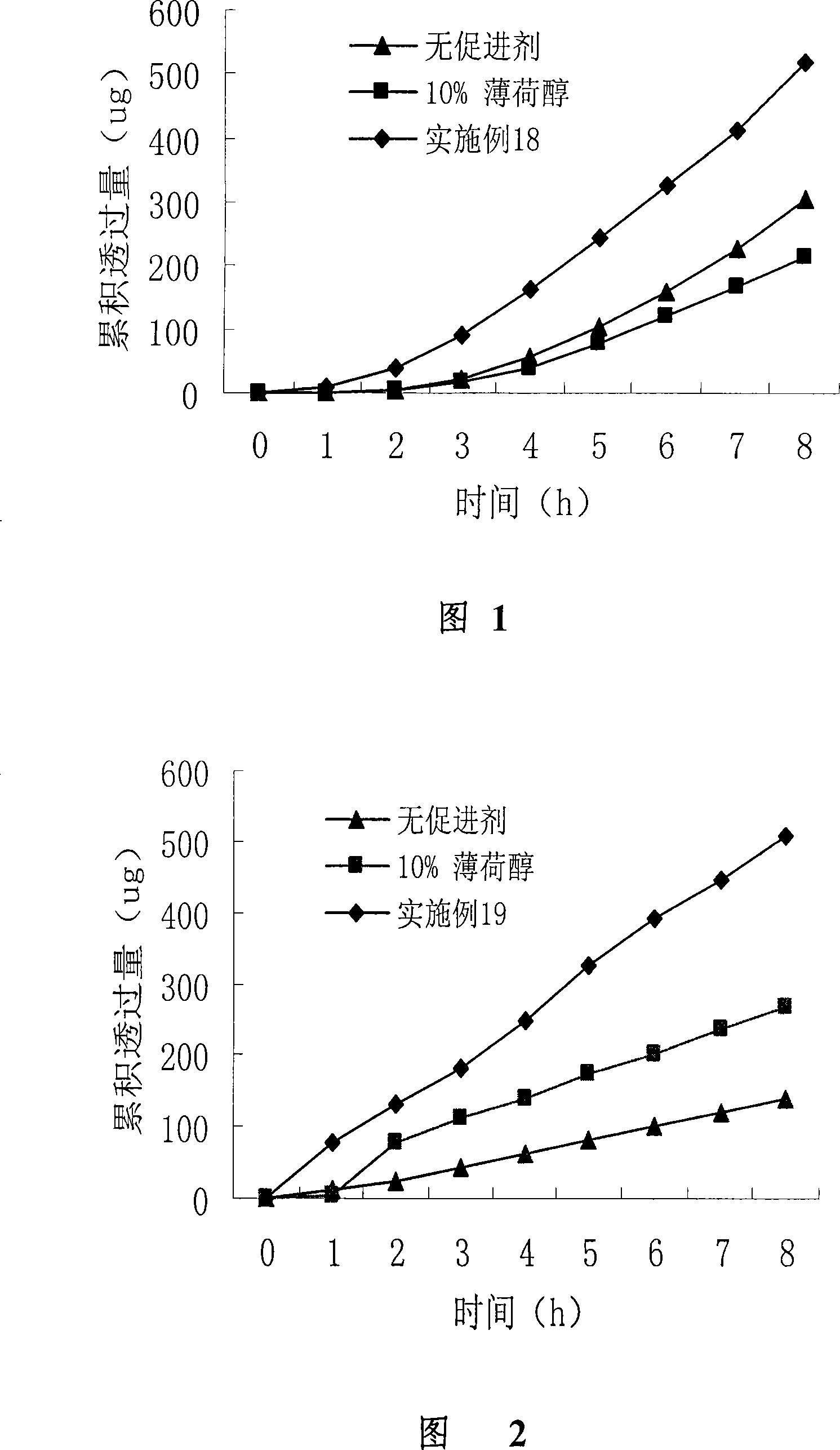
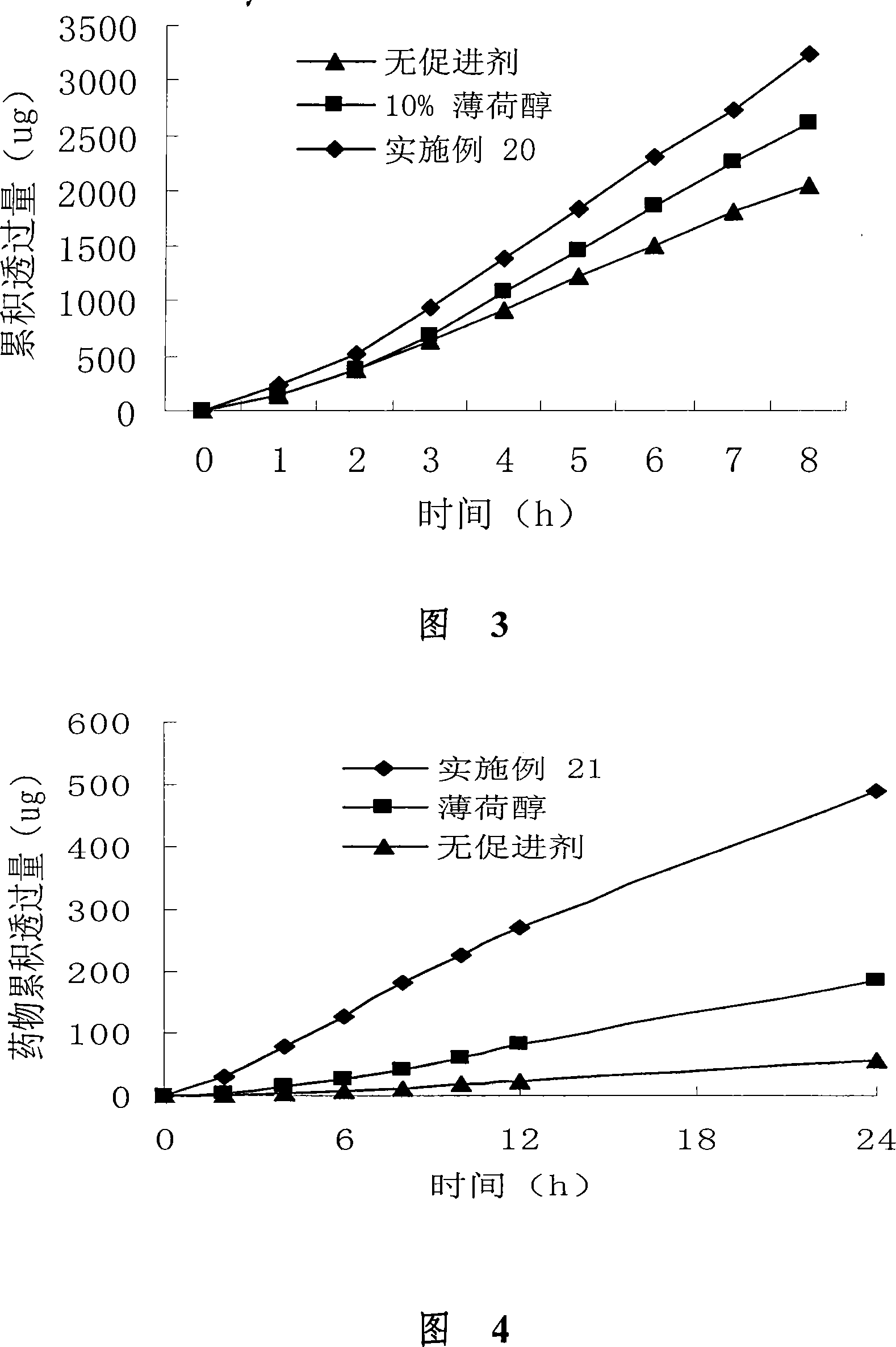
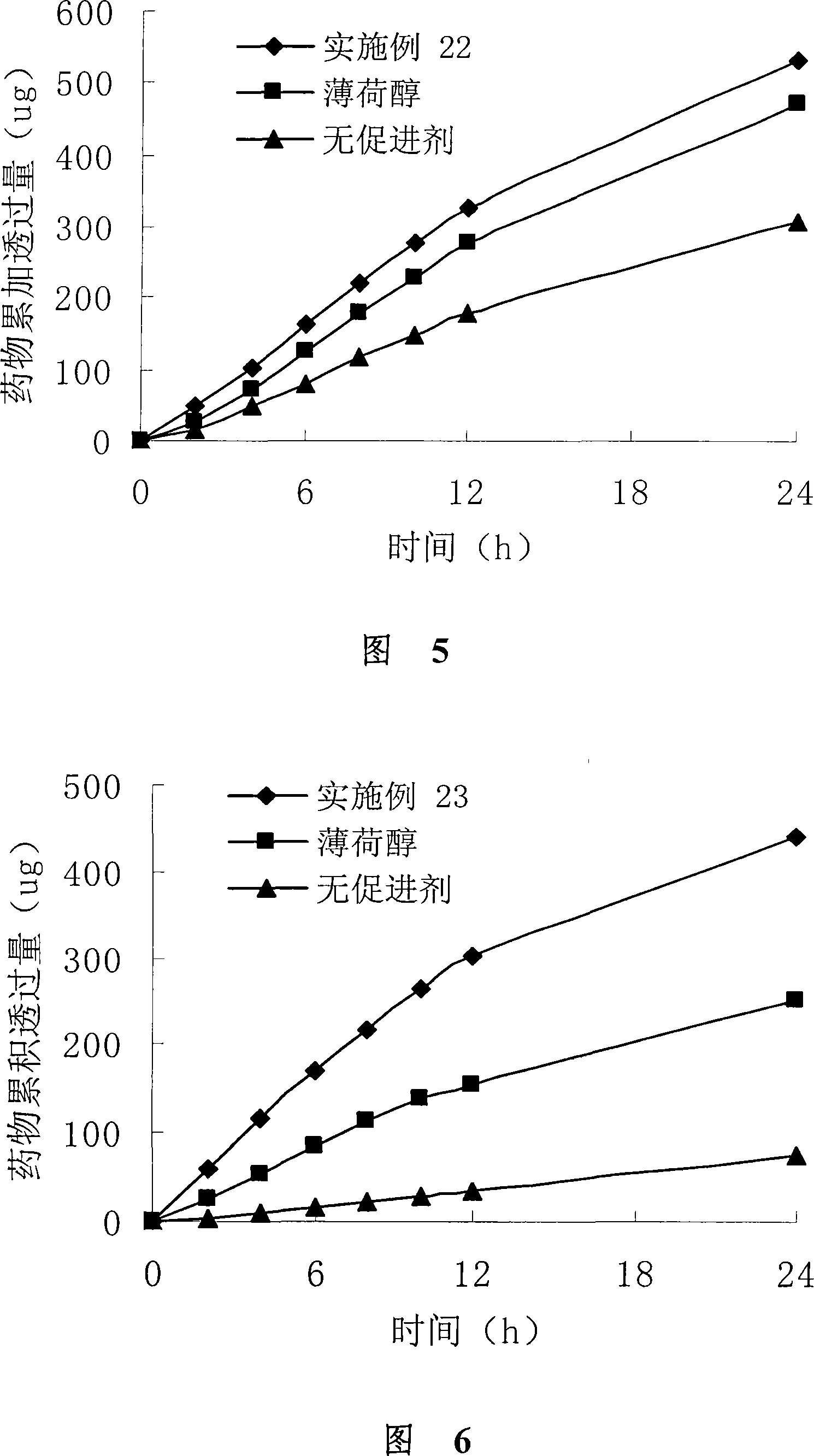
Image
Examples
Embodiment 1
[0039] Take 12g (0.2mol) of acetic acid, add 17.85g (0.15mol) of thionyl chloride, mix and react at 60°C for 3 hours, add 20ml of menthol tetrahydrofuran solution containing 15.6g (0.1mol), and continue the reaction for 3 hours. The reaction solution was adjusted to pH 6-7 with NaOH solution, the organic layer was separated, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, washed with saturated brine, and anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, perform column chromatography (silica gel is 200-300 mesh), use petroleum ether and ethyl acetate (15:1) as the eluent, and evaporate the eluent to obtain 18.64 g of a colorless liquid. Rate: 94%. product of 1 HNMR and MS data are as follows: 1 HNMR (CDCl 3 ), δ: 0.71 (3H, d, J = 7.0Hz, Me-2-isopropyl), 0.83 (3H, d, J = 2.2Hz, Me-2-isopropyl), 0.85 (3H, d, J = 1.6Hz , Me-5), 0.96~1.05(3H, m), 1.33~1.45(2H, m), 1.73~1.83(2H, m), 1.91~1.92(2H, m), 1.96(3H, s, Me- ester), 4.68 (1...
Embodiment 2
[0041] Take 14.8g (0.2mol) of propionic acid, add 17.85g (0.15mol) of thionyl chloride, mix and react at 60°C for 3 hours, add 20ml of tetrahydrofuran solution containing 15.6g (0.1mol) of menthol, and continue the reaction for 3 hours, the reaction solution was adjusted to pH 6-7 with NaOH solution, the organic layer was separated, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, washed with saturated brine, anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, perform column chromatography (silica gel is 200-300 mesh), use petroleum ether and ethyl acetate (15:1) as the eluent, and evaporate the eluent to obtain 19.32 g of a colorless liquid. Rate: 91.4%. product of 1 HNMR and MS data are as follows: 1 HNMR (CDCl 3 ), δ: 0.76 (3H, d, J = 7.0Hz, Me-2-isopropyl), 0.89 (3H, d, J = 2.2Hz, Me-2-isopropyl), 0.91 (3H, d, J = 1.6Hz , Me-5), 0.99~1.04(2H, m), 1.07~1.11(1H, m), 1.14(3H, t, J=7.6Hz, Me-ester), 1.33~2.00(6H, m), 2.30 (2H, q...
Embodiment 3
[0043] Take 17.6g (0.2mol) of butyric acid, add 17.85g (0.15mol) of thionyl chloride, mix and react at 60°C for 3 hours, add 20ml of tetrahydrofuran solution containing 15.6g (0.1mol) of menthol, and continue the reaction for 3 hours, the reaction solution was adjusted to pH 6-7 with NaOH solution, the organic layer was separated, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, washed with saturated brine, anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, and perform column chromatography (silica gel is 200-300 mesh), using petroleum ether and ethyl acetate (15:1) as the eluent, and evaporate the eluent to obtain 20.91 g of a colorless liquid. For: 92.4%. product of 1 HNMR and MS data are as follows: 1 HNMR (CDCl 3 ), δ: 0.76 (3H, d, J = 6.9Hz, Me-2-isopropyl), 0.89 (3H, d, J = 2.2Hz, Me-2-isopropyl), 0.91 (3H, d, J = 1.6Hz , Me-5), 0.97~1.11(6H, m), 1.13~1.15(2H, m), 1.59~1.69(4H, m), 1.83~1.87(1H, m), 1.92~1.98(1H, m) , 2.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com