Selective apoptotic induction in cancer cells including activation of procaspase-3
A kind of caspase enzyme, caspases technology, applied in the direction of organic chemistry and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1. Caspase zymogen activating compounds
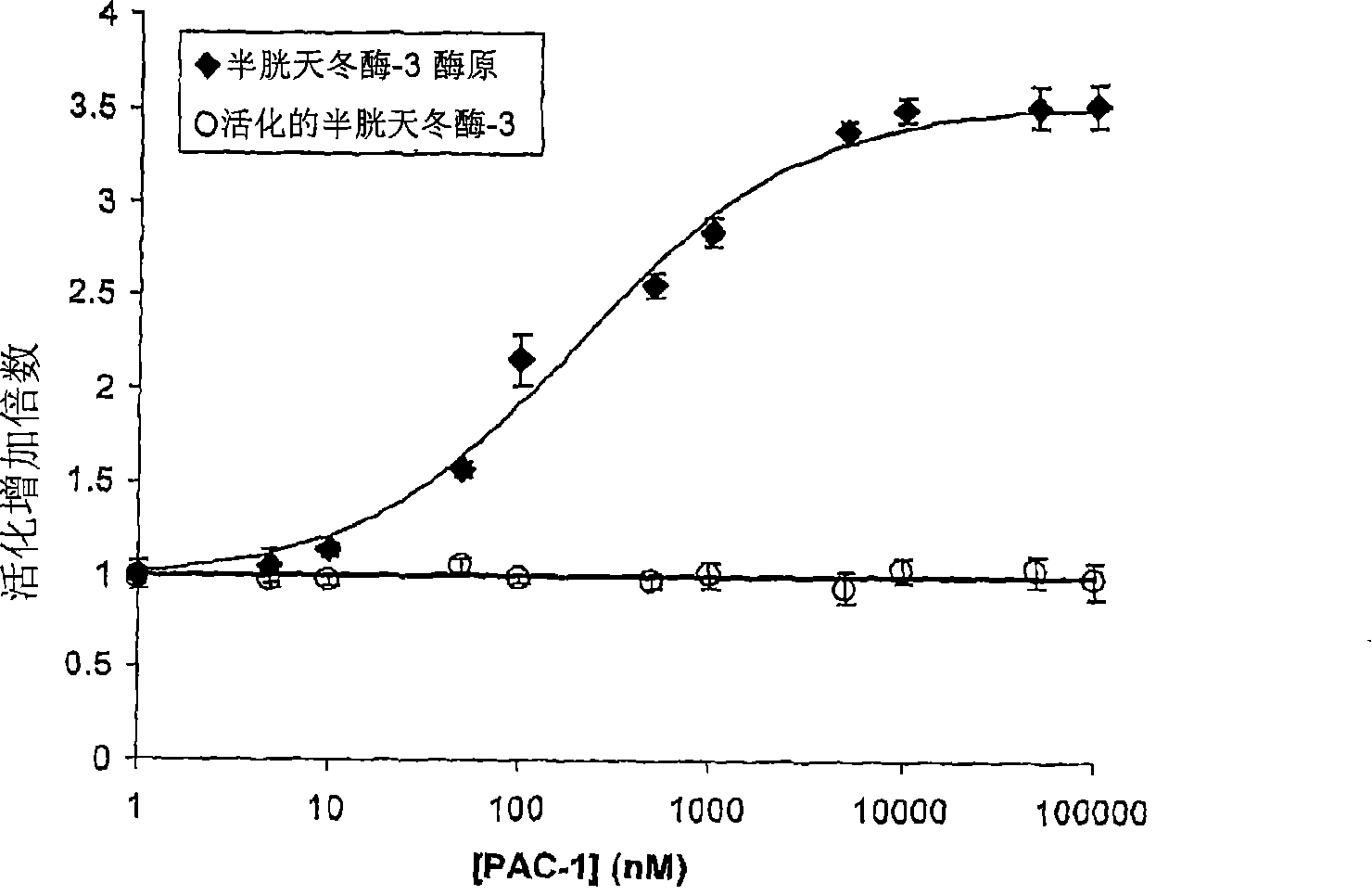
[0103] Mutations or aberrant expression of proteins involved in the apoptotic cascade are common hallmarks of cancer. These changes prevent the transmission of pro-apoptotic signals to the executor caspases, thereby preventing apoptosis-induced cell death and allowing cell proliferation. Caspase-3 and caspase-7 are key executor caspases that exist as inactive zymogens activated by upstream signals. Importantly, procaspase-3 expression levels were significantly higher in certain cancer cells than in noncancerous control cells. We report here the identification of small molecules that directly activate the pro-caspase-3 zymogen to active caspase-3. The specific compound PAC-1 acts to activate in vitro, EC 50 is approximately 220 nmol, and this compound induces apoptosis in a variety of cancer cell lines.
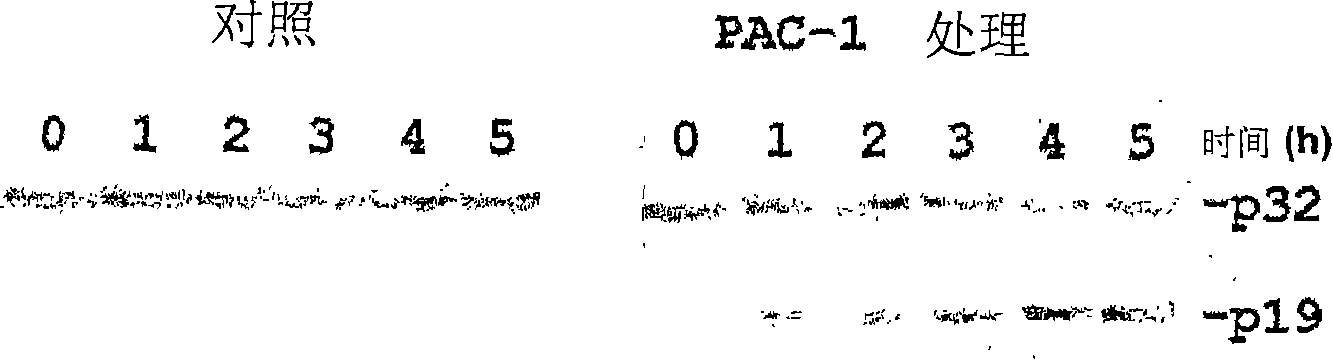
[0104] Unlike many known anticancer drugs, cells treated with PAC-1 exhibited immediate activation of procaspase-3...
Embodiment 2
[0139] Example 2. Synthesis of pro-caspase activating compounds.
[0140] PAC-1 and other compounds were prepared according to the following schemes such as Scheme 1 and / or Scheme 2. Other variants can be prepared according to methods known in the art.
[0141]
[0142] plan 1
[0143] In a specific example, PAC-1 was prepared according to Scheme 2:
[0144]
Embodiment 3
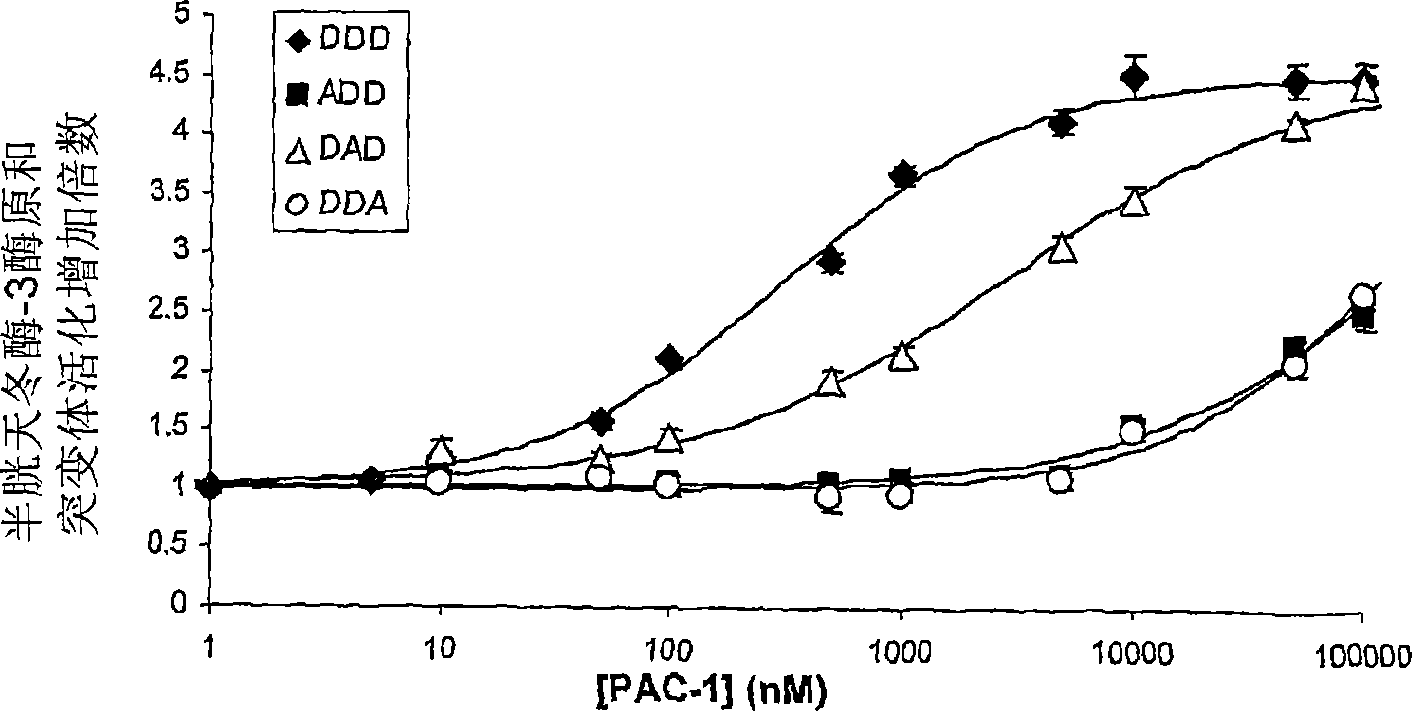
[0145] Example 3. Analogs of PAC-1.
[0146] Analogous compounds of PAC-1 were prepared and evaluated for their ability to directly activate purified procaspase-3 zymogen in vitro.
[0147] Table 2. Activity of PAC-1 and similar compounds.
[0148]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com