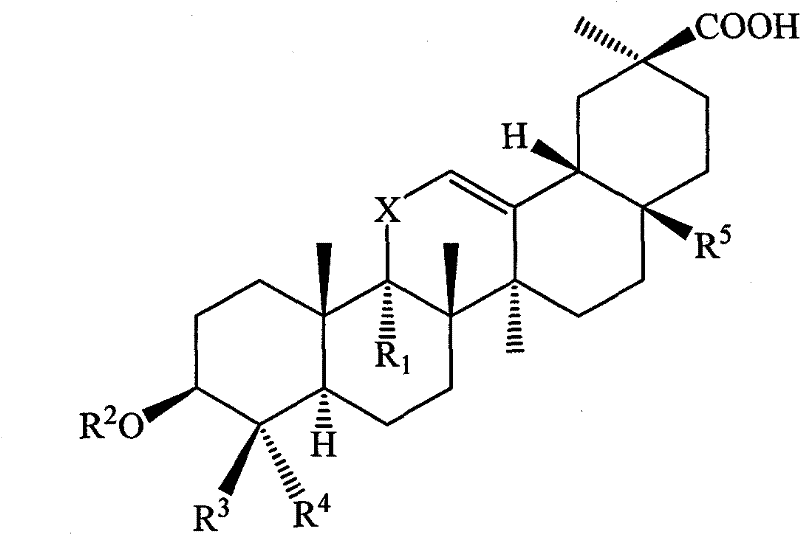
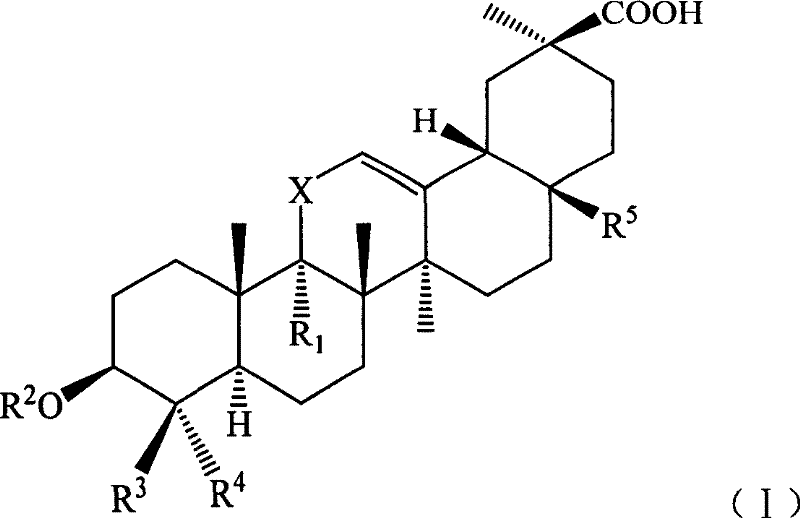
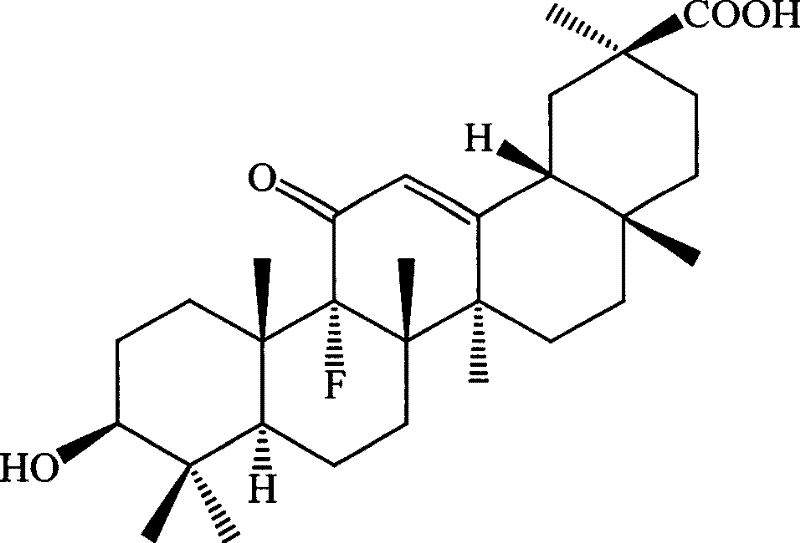
Compound with liver-protecting activity
A compound and drug technology, applied in the field of compounds with hepatoprotective activity, can solve problems such as difficult to remove viruses, liver tissue damage, abnormal liver function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0146] Example 13 Preparation of β-hydroxy-9-fluoro-11-oxo-18β, 20β-oleanane-12-enoic acid
[0147] Step 1: In a dry reaction flask, add 30ml each of acetic anhydride and pyridine, add 4.7g (10mmol) 3β-hydroxyl-11-oxidation-18β, 20β-oleanane-12-enoic acid after stirring, at 60 Stir the reaction at ℃ for 10 h, after the reaction is completed, pour the reaction solution into ice water under vigorous stirring, and a yellow solid precipitates, dry at 60 °C and then add it to the reaction flask, add 50 ml of absolute ethanol and 1 ml of concentrated sulfuric acid, heat and stir to reflux for 5 h, After the reaction is completed, the solvent is recovered, the residue is added with an appropriate amount of ice water and extracted with ethyl acetate 50ml×2, the organic layers are combined and dried, and the solvent is recovered to obtain 3β-acetoxy-11-oxidized-18β,20β-oleanane -12-enoic acid ethyl ester 4.1g, yield: 75.9%.
[0148] Step 2: Add 4.0 g (7.4 mmol) of 3β-acetoxy-11-oxid...
Embodiment 2
[0150] Example 2 Preparation of 3β-hydroxy-9-chloro-11-oxo-18β, 20β-oleanane-12-enoic acid
[0151] Step 1: Same as Step 1 in Example 1.
[0152] Step 2: Add 4.0g (7.4mmol) of ethyl 3β-acetoxy-11-oxidation-18β,20β-oleanane-12-enoate and carbon tetrachloride to a dry reaction flask under a nitrogen atmosphere 50ml, 10mlSO 2 Cl 2 / carbon tetrachloride 50ml, heat up to 60°C and stir for 3h, evaporate the solvent under reduced pressure; after cooling down to room temperature, add 50ml of water, then extract with 30ml×3 ethyl acetate, combine the organic phases and wash with saturated sodium bicarbonate solution , dried and filtered, concentrated under reduced pressure, the residue was dissolved in 95% ethanol, 0.9g of sodium hydroxide was added, and after reflux and stirring for 1h, the solvent was recovered under reduced pressure at 50°C, the residue was added with 50ml of water, and the pH was adjusted to 4 with dilute hydrochloric acid. ~6, a light yellow solid was precipi...
Embodiment 3
[0154] Example 3 3-(β-2-O-β-D-glucopyranuronyl-α-D-glucopyranuronic acid)-9-fluoro-11-oxo Preparation of -18β, 20β-oleanane-12-enoic acid
[0155]Referring to the preparation method in Example 1, 3β-hydroxy-11-oxidized-18β,20β-oleanane-12-enoic acid was replaced with 3-(β-2-O-β-D-glucopyranoside Aldo-α-D-glucopyranurosidic acid)-11-oxo-18β, 20β-oleanane-12-enoic acid, yielding 3-(β-2-O-β-D- Glucopyranurosidyl-α-D-glucopyranuronic acid)-9-fluoro-11-oxo-18β, 20β-oleanane-12-enoic acid 2.8g, yield: 55.1% .
[0156] Elemental analysis (C 42 h 61 FO 16 ): C: 59.85%, H: 7.42%, F: 2.15% (theoretical: C: 59.99%, H: 7.31%, F: 2.26%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com