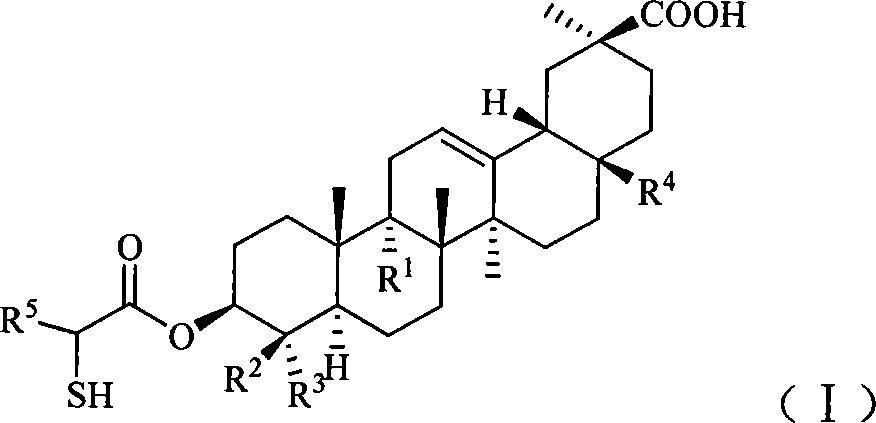
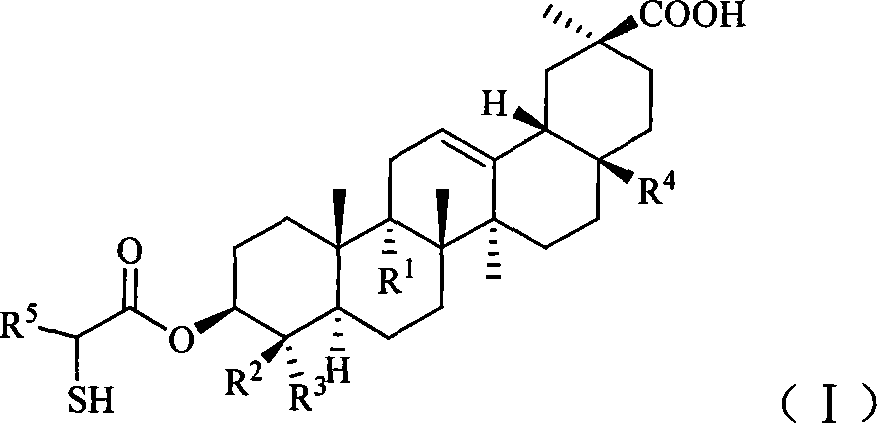
Compound used for liver disease
A technology for compounds and stereoisomers, applied in the field of pharmaceutical compositions, can solve problems such as poor hydrophilicity and poor oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
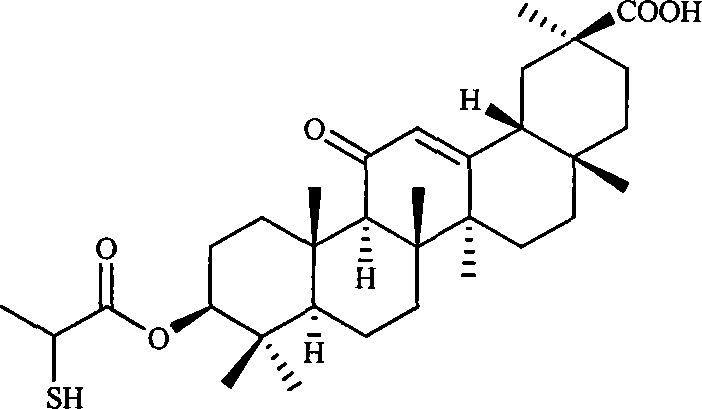
Embodiment 13
[0124] The preparation of embodiment 13-(2-mercapto-propionyl)-11-oxo-18β, 20β-oleanane-12-enoic acid
[0125] In a dry reaction flask, add 50ml of acetonitrile, 5g (10.6mmol) of 3β-hydroxy-11-oxidized-18β,20β-oleanane-12-enoic acid, and then add 5ml of triethylamine, stir to dissolve and heat up to reflux , and then drop 2-mercapto-propionyl chloride 1.9g (15mmol) / 20ml acetonitrile solution, continue to stir and reflux for 10h after the dropwise addition, recover the solvent, add an appropriate amount of ice water to the residue and extract it with ethyl acetate 50ml×2, organic The layer was dried, and the solvent was recovered to obtain 4.8 g of 3-(2-mercapto-propionyl)-11-oxo-18β,20β-oleanane-12-enoic acid, yield: 81.2%.
[0126] Elemental analysis (C 33 h 50 o 5 S):
[0127] C: 70.74%, H: 9.09%, S: 5.69%
[0128] Theoretical: C: 70.93%, H: 9.02%, S: 5.74%.
Embodiment 2
[0129] Example 2 Preparation of 3-(2-mercapto-propionyl)-9-fluoro-11-oxo-18β,20β-oleanane-12-enoic acid
[0130] Step 1: In a dry reaction flask, add 30ml each of acetic anhydride and pyridine, add 4.7g (10mmol) 3β-hydroxyl-11-oxidation-18β, 20β-oleanane-12-enoic acid after stirring, at 60 Stir the reaction at ℃ for 10 h, after the reaction is completed, pour the reaction solution into ice water under vigorous stirring, and a yellow solid precipitates, dry at 60 °C and then add it to the reaction flask, add 50 ml of absolute ethanol and 1 ml of concentrated sulfuric acid, heat and stir to reflux for 5 h, After the reaction is completed, the solvent is recovered, the residue is added with an appropriate amount of ice water and extracted with ethyl acetate 50ml×2, the organic layers are combined and dried, and the solvent is recovered to obtain 3β-acetoxy-11-oxidized-18β,20β-oleanane -12-enoic acid ethyl ester 4.1g, yield: 75.9%.
[0131] Step 2: Add 4.0 g (7.4 mmol) of 3β-ac...
Embodiment 3
[0136] Example 3 Preparation of 3-(2-mercapto-propionyl)-9-chloro-11-oxo-18β,20β-oleanane-12-enoic acid
[0137] Step 1: Same as Step 1 in Example 2.
[0138] Step 2: Add 4.0g (7.4mmol) of ethyl 3β-acetoxy-11-oxidation-18β,20β-oleanane-12-enoate and carbon tetrachloride to a dry reaction flask under a nitrogen atmosphere 50ml, 10mlSO 2 Cl 2 / carbon tetrachloride 50ml, heat up to 60°C and stir for 3h, evaporate the solvent under reduced pressure; after cooling down to room temperature, add 50ml of water, then extract with 30ml×3 ethyl acetate, combine the organic phases and wash with saturated sodium bicarbonate solution , dried and filtered, concentrated under reduced pressure, the residue was dissolved in 95% ethanol, 0.9g of sodium hydroxide was added, and after reflux and stirring for 1h, the solvent was recovered under reduced pressure at 50°C, the residue was added with 50ml of water, and the pH was adjusted to 4 with dilute hydrochloric acid. ~6, a light yellow soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com