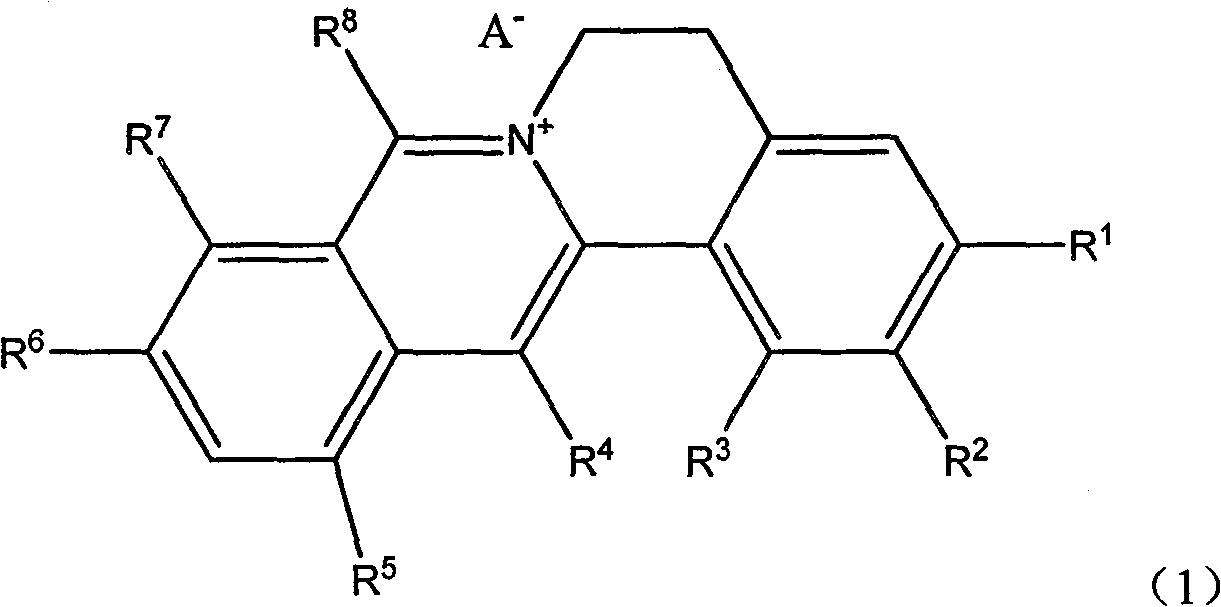
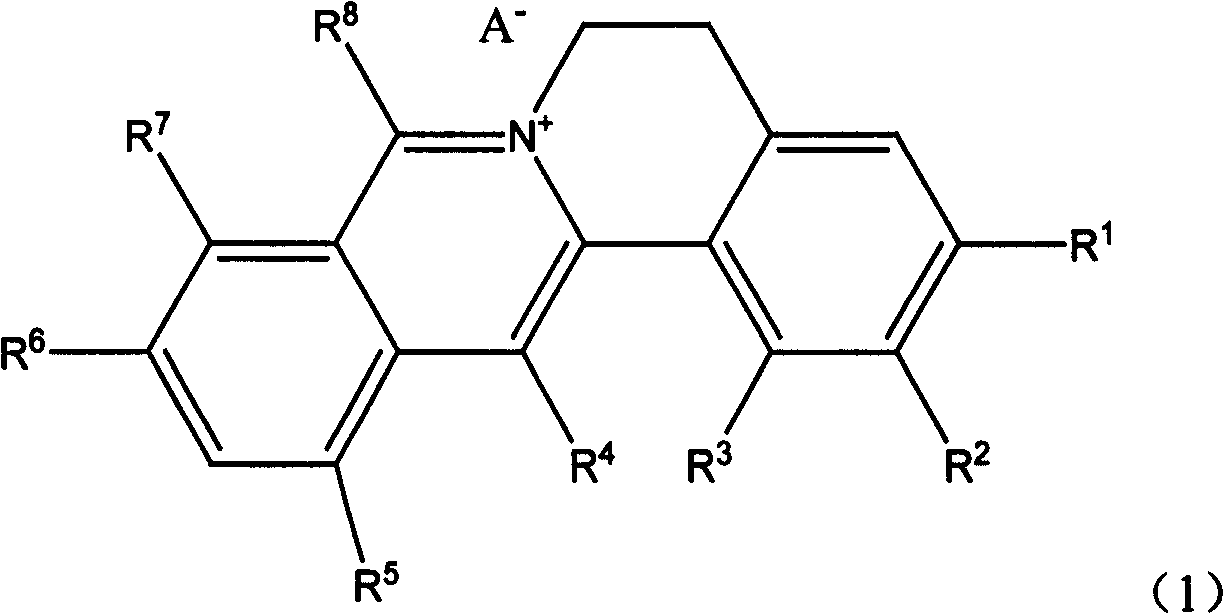
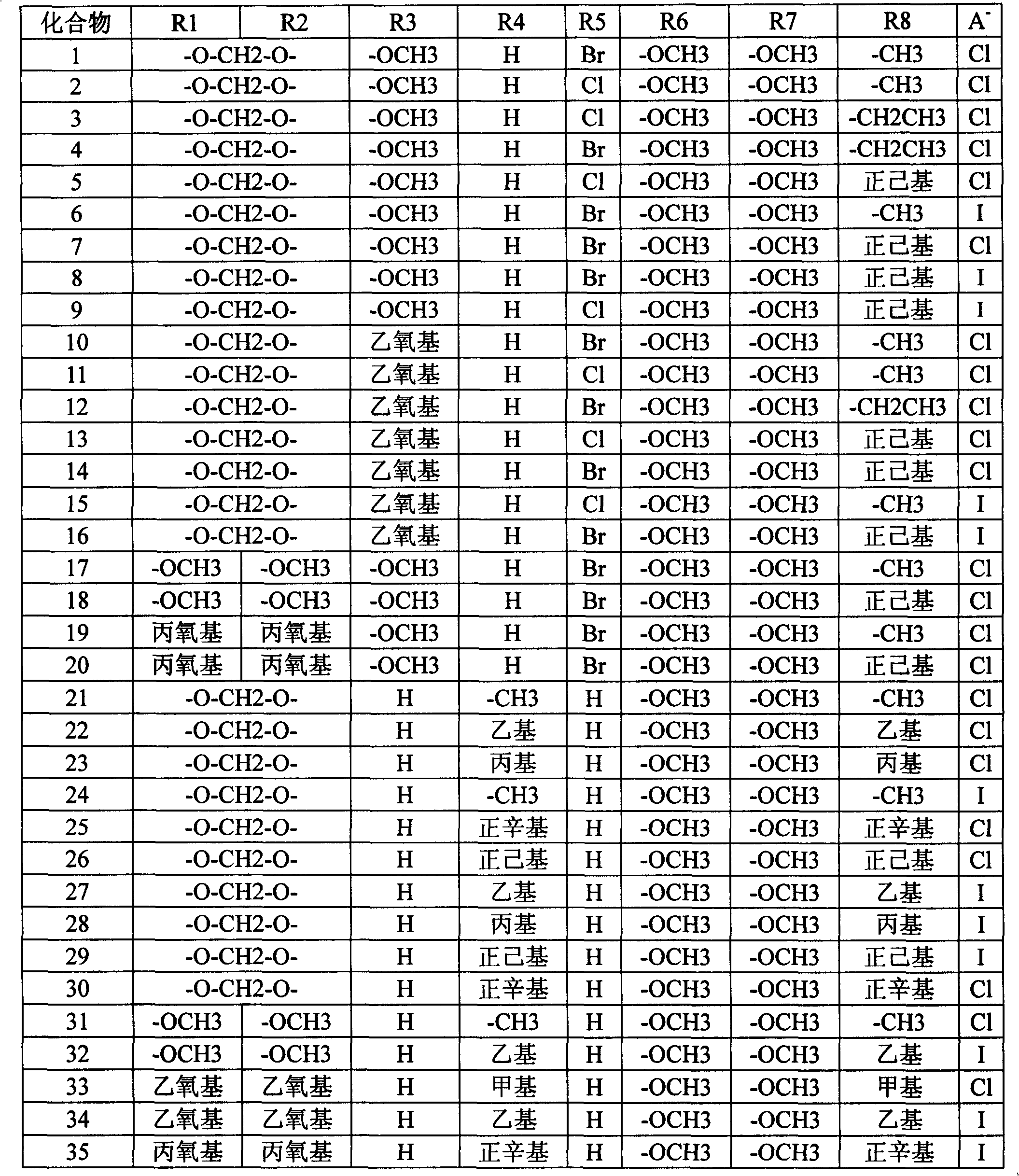
Quinolizine derivatives having antibacterial activity
A compound, methoxy technology, applied in the field of medicine, can solve problems such as irregular use, multi-drug resistance, and clinical treatment difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1 Compound 1 (R 1 -R 2 Methylenedioxy (-O-CH 2 -O-), R 3 is methoxy, R 4 for hydrogen, R 5 is bromine, R 8 is methyl, R 6 、R 7 is methoxy, A - Chloride ion, namely 1-methoxy-8-methyl-12-bromo-berberine chloride) preparation of
[0109] Reaction equation:
[0110]
[0111] Reaction steps:
[0112] (1) 37.2g (100mmol) of berberine hydrochloride and 200ml of anhydrous tetrahydrofuran (THF moisture is less than 0.03%) are added in the dry reaction flask, heated and stirred to reflux, and anhydrous tetrahydrofuran solution of 15g of methyl iodide is added dropwise. After reflux for 5 hours, the reaction solution was concentrated and purified by silica gel chromatography (eluted with chloroform / ethyl acetate (15:1)) to obtain 8-methylberberine iodide.
[0113] (2) Dissolve 8-methylberberine iodide in 100ml of ice water, stir to dissolve, add bromine 16g (200mmol) dropwise at 20°C, after the drop is complete, stir...
Embodiment 2
[0121] Example 2 Compound 7 (R 1 -R 2 Methylenedioxy (-O-CH 2 -O-), R 3 is methoxy, R 4 is methyl, R 5 is bromine, R 8 is n-hexyl, R 6 、R 7 is methoxy, A - Chloride ion, namely 1-methoxy-8-n-hexyl-12-bromo-berberine chloride compound) preparation
[0122]
[0123] Reaction steps: (1) In a dry reaction flask, add 0.5 grams of magnesium powder, 50 ml of anhydrous tetrahydrofuran (THF moisture is less than 0.03%), under stirring, add a little iodine, dropwise add 1-bromo-n-hexane in anhydrous tetrahydrofuran A few drops until the color of iodine completely fades, add 3.3g of 1-bromo-n-hexane (0.02mol) in anhydrous tetrahydrofuran solution dropwise at a rate that keeps the reaction solution slowly refluxed, after the addition, reflux for 1 hour, and add 7.5g of berberine hydrochloride (0.02mol), and then reflux for 2h, the reaction solution was concentrated, and purified by silica gel chromatography (eluted with chloroform / ethy...
Embodiment 3
[0132] Example 3 Compound 21 (R1-R2 is methylenedioxy (-O-CH2-O-), R3 is hydrogen, R4 is methyl, R5 be hydrogen, R8 is methyl, R6, R7 are methoxy, A- is chloride ion, i.e. 8, the preparation of 13-dimethylberberine chloride) Reaction equation:
[0133]
[0134] Reaction steps:
[0135] (1) Add 15g (40mmol) of berberine hydrochloride and 80ml of 20% acetone aqueous solution into the reaction flask, stir and dissolve, then slowly add 40ml of 50% aqueous sodium hydroxide solution dropwise, after the addition is completed, stir vigorously for 1h, and a large amount of orange yellow is precipitated solid. Filter and wash with 20% acetone. After the filter cake is dried, dissolve it with 100ml of dry acetonitrile, add 15ml of 1-iodomethane (d=2.29), and react the mixture under reflux for 3 hours, then concentrate to obtain 13-methylberberine iodide, which can be directly carried out to the next step without purification .
[0136] (2) In a dry reaction bottle, add 50ml of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com